|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Miessler G., Tarr D.A. — Inorganic Chemistry |
|
|
 |
| Предметный указатель |
Heteroboranes 577 579
Heterogeneous catalysis 534 548—571
Heteronuclear diatomic molecules 134 135 138
Hexaamminecobalt(III) chloride 300
Hexagonal close packing (hep) 210 211 213
HF 287
HF, symmetry 89
HI 287
High spin complexes 347 348 351 367 368
High symmetry groups 82—85
Highest Occupied Molecular Orbital (HOMO) 127 137
Highest order rotation axis 78
Hinze, J., electronegativity 64
History of inorganic chemistry 5 11
Hoffmann, R. 558 559 561 565
Hole formalism 406
Holes (electron vacancies) 223 225—227
Holes, octahedral and tetrahedral in crystals 210 212
HOMO (highest occupied molecular orbital) 127 137
HOMO (highest occupied molecular orbital) and ionization energy 187
HOMO (highest occupied molecular orbital),  138 138
HOMO (highest occupied molecular orbital),  153 154 156 170 153 154 156 170
HOMO-LUMO, combination and acid-base reaction 171—174 176
HOMO-LUMO, diagrams 173 174 177 187
HOMO-LUMO, energies and hydrogen bonding 177
HOMO-LUMO, interactions 173 174 177 187
Homogeneous catalysis 534 535 537—539 541 543 544 547 549 550
Homogeneous catalysis, water gas shift reaction 550
Homoleptic compounds 476
Homonuclear diatomic molecules 116 122 125—128 130 132 133
HSAB and qualitative analysis 185
HSAB and solubility 222
HSAB, halogens as examples 188
Huheey, J.E., electronegativity 64 65
Hund’s rules 386 388
Hund’s rules, maximum multiplicity 35 386
Hybrid orbitals 158 159
Hybrid orbitals and group theory 157—161
Hybrid orbitals,  159 160 159 160
Hybrid orbitals,  , , 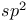 158 158
Hybrid orbitals, water 158
Hydrate isomerism 309 310 319
Hydrated metal ion acidities 198
Hydration enthalpies of  transition metal ions, LFSE 351 352 transition metal ions, LFSE 351 352
Hydration enthalpies of  transition metal ions, simulated 375 transition metal ions, simulated 375
Hydrazine 274
Hydrazine, oxidation 275
Hydrazoic acid  274 274
Hydride complexes 477
Hydride elimination 533
Hydride ion 248
Hydrides 248
Hydrofluoric acid 203
Hydroformylation 535 537 538
Hydrogen 247
Hydrogen as a fuel 248
Hydrogen atom wave functions, angular functions 26
Hydrogen atom wave functions, radial functions 27
Hydrogen atom, energy levels 20
Hydrogen atom, spectrum 21
Hydrogen atoms, bridging 1 3 256 257 259
Hydrogen atoms, terminal 1 3
Hydrogen bonding 174 176
Hydrogen bonding and molecular orbitals 174 176 177
Hydrogen bonding in ice 71
Hydrogen bonding in proteins 71 72
Hydrogen bonding, unsymmetrical, molecular orbitals 176
Hydrogen burning 6
Hydrogen fluoride, boiling point 69
Hydrogen ion 248
Hydrogen isotopes 6 9 247
Hydrogen peroxide decomposition rates 600
Hydrogen sulfide  bond angle 66 bond angle 66
Hydrogen, bridging 256 257 259
Hydrogen, chemical properties 248
Hydrogen, preparation 248
Hydrogen-bonded protein structures,  helix 71 72 helix 71 72
Hydrogen-bonded protein structures, pleated sheet 71 72
Hydrogenase enzymes 475
Hydrogenation, by Wilkinson’s catalyst 542—544
Hydrohalic acids 287
Hydrohalic acids, carbonyl complex parallels 557
Hydrolysis of esters, amides, and peptides 446
Hydronium ion 167
Hydroxyl radical, photochemical smog 631
Hydroxymethylation, by cobalamin 603
I (internal) strain 199
i, inversion operation 79
Ice, structure 71
Identity operation (E) 77 81 93
Imperfections in Solids 231 232
Improper rotation 79 81
Indium, properties 260
Inductive effects 196
Industrial chemicals, top twenty 240
Inert pair effect 260 271
Infrared spectra 76 103 104 106—110
Infrared spectra and acid-base reactions 192
Infrared spectra, carbonyl stretching bands 506
Infrared spectra, infrared-active molecular vibrations 76 103 104 106—110
Infrared spectra, number of infrared bands 503
Infrared spectra, organometallic compounds 503 504 506
Ingold — Robinson acid-base definition 166
Inner and outer orbital complexes 343
Inorganic compounds, examples of geometries 3
Insertion reactions 1,1 527
Insertion reactions 1,2 527 528 532
Insulator, band structure 223
Interchange mechanism (I) 415
Interchange mechanism (I) in square-planar reactions 435 436
Interchange mechanism (I), rate equation 417—419
Interhalogen compounds 289
Interhalogen compounds, carbonyl parallels 557
Intraligand bands 408
Intrinsic semiconductor 225
Inverse of a group operation 93
Inversion (i) 79 81
Iodine adduct colors 178 180
Iodine, isolation 285
Ion exchange properties of zeolites 236
Ionic compounds and molecular orbitals 138 139
Ionic crystals 138
Ionic radius 44 46 47 668—671
Ionic radius and ionic charge 47
Ionic radius and nuclear charge 47
Ionic radius and number of electrons 47
Ionic radius, reaction rates 422
Ionization energy 43 44 138 139 244 671 672
Ionization energy, Born — Haber cycle 220
Ionization isomerism 309 310 320
Ionization potential 43
IR spectra, active vibrational modes 76 103 104 106—110
IR spectra, CO complexes 109 110 468—471 504 506
Iron pyrites  in mine tailings 629 in mine tailings 629
Irreducible representation 96—102 105—107
Irreducible representation, characters of 96
Irreducible representation, notation 101 102
Isocarbonyls (oxygen-bonded carbonyls) 474
Isoelectronic molecules 52 156
Isolobal  and CR 561 and CR 561
Isolobal  and and  561 561
Isolobal analogy 558—561 563—566
Isolobal analogy, extensions 561 565
Isolobal definitions 558—561
Isolobal symbol 559
Isomerism, coordination compounds 309—313 315 316 318—320 322 323
Isomerization by cobalamin 603
Isomerization of chelate rings 433
Isomerization, twist mechanisms 434
Isomers of triaminotriethylamine complexes 312
| Isomers, ![$[Co(tren)(sal)]^+$](/math_tex/565f1a38dc73cf91922f4f04dcfeaa0782.gif) 313 313
Isomers, 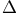 316 316
Isomers, 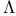 316 316
Isomers, 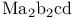 314 314
Isomers, ambidentate 309 320 321
Isomers, chelate ring combinations 315—317
Isomers, chiral 310
Isomers, cis and trans 302 304 308 310 311
Isomers, cis and trans, diamminedichloroplatinum (II), ![$\mathrm{[PtCl_2(NH_3)_2]}$](/math_tex/cf8ec176e2dfb7c906e31ae8f11e9e4d82.gif) 308 308
Isomers, classification 310
Isomers, conformational 310
Isomers, constitutional 310
Isomers, coordination 309 320
Isomers, facial 312
Isomers, four-coordinate complexes 310 311
Isomers, geometric 310
Isomers, hydrate 309 310 319
Isomers, identification 322
Isomers, ionization 309 310 320
Isomers, linkage 309 310 320 321
Isomers, meridional 312
Isomers, number for specific complexes 315
Isomers, optical 302 310
Isomers, separation 322
Isomers, six-coordinate complexes 311—313 315
Isomers, solvent 309
Isomers, stereo 309 310
Isomers, structural 310
Isomers, X-ray crystallography for identification 322
Isomers, [Mabcdef] 313
Isotopes, definition 8
Isotopes, hydrogen 247
Jaffe, H.H., electronegativity 64
Jahn — Teller, distortion 370 398 400
Jahn — Teller, distortions and spectra 398
Jahn — Teller, effect 327 370—372 398
Jahn — Teller, excited states 400
Jahn — Teller, theorem 398
Jean, Y. 162
Jorgensen, S.M., coordination chemistry 12 300 301
Kaim, W. 635
Kammerling Onnes, H 228
Kaolinite 234
Kealy, T.J. 457
Kekule, F.A. 16
Kettle, S.F.A. 110
KF as base 168
Kinetic chelate effect 428
Kinetic consequences of reaction pathways 417
Kinetics and stereochemistry of square-planar substitutions 434
Klado borane definition 574
Klechkowsky’s rule 37
Krypton, fluoride compounds 295
Krypton, isolation 291
Kyoto, Japan, conference on greenhouse gases 268
l, angular momentum quantum number 26—28
L, quantum number 340
Laccase 595
Lactotransferrin 604
Lanthanide contraction 49
Lanthanides 17
Laporte selection rule 390 406
Latimer diagrams 676—680
Latimer diagrams, hydrogen 245
Latimer diagrams, nitrogen 278
Latimer diagrams, oxygen 246
Lattice energy 220 221
Lattice enthalpy 139 221
Lattice enthalpy and Madelung constant 220 221
Lattice enthalpy, Born — Haber cycle 220
Lattice points 209
Lavoisier, acid-base definition 166
Lead 262
Lead in paint 627
Lead in tetraethyllead, 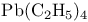 627 627
Lead, environmental 627
Lead, toxicity 262
Leaving groups, effect on rate 435—437
Leveling effect and solvent properties 201 202
Levorotatory 102
Lewis acid-base definition 166 170
Lewis acids, and carbyne complexes 56 501
Lewis bases, disproportionation by 57 557
Lewis model of 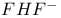 143 143
Lewis, G.N 170
Lewis, G.N, electron-dot diagrams 51—53 55—61 691—695
LFSE (ligand field stabilization energy) 345 348 349 351
LFSE (ligand field stabilization energy) and  bonding 355 356 bonding 355 356
LFSE (ligand field stabilization energy) and water exchange rate 421
LFSE (ligand field stabilization energy) of hydration 351 352
LFSE (ligand field stabilization energy), calculation 351
Liebig, J. and ethylene complexes 457
Liebig, J., acid-base definition 165 166
Ligand bulk and reactivity 523
Ligand close-packing (LCP) 66
Ligand cone angle 523
Ligand dissociation reactions 520—522
Ligand field activation energy (LFAE) 420
Ligand field stabilization energy (LFSE) 345 349 351
Ligand field stabilization energy (LFSE) for aqueous ions 349
Ligand field stabilization energy (LFSE),  bonding 355 bonding 355
Ligand field stabilization energy (LFSE), calculation 351
Ligand field stabilization energy (LFSE), enthalpy of hydration 351 352
Ligand field strength and spin states 348
Ligand field theory 12 304 342 345
Ligand reducibility and electron transfer 444
Ligand ring conformation 318 319
Ligand substitution 521 522
Ligand to metal   bonding 355 bonding 355
Ligand to metal charge transfer (LMCT) 407
Ligands 302
Ligands,  acceptor 364 365 368 acceptor 364 365 368
Ligands,  donor 366—368 donor 366—368
Ligands,  donor and spectra 367 donor and spectra 367
Ligands, ambidentate 320
Ligands, bidentate 307
Ligands, bridging 308 460
Ligands, chelating 302
Ligands, common monodentate 305
Ligands, common multidentate 306
Ligands, defined 299
Ligands, organometallic 462 467
Ligands, organometallic compounds 459
Ligands, reactions of coordinated 446 448
Ligands, strong field 346 347 351 367
Ligands, weak field 346 347 351 367
Light-activated switch 227
Light-emitting diode (LED), emission frequencies 227
Linear  systems 479 480 482—484 systems 479 480 482—484
Linear combinations of the atomic orbitals (LCAO) 116
Linear free energy relationship (LFER) 423 424
Linear geometry, VSEPR 58 61—63
Linkage (ambidentate) isomerism 309 310 320
Lippard, S.J. 635
Lithium aluminum hydride 248
Lithium halide solubilities 181
Lithophiles 10
London forces 69
Lone pair repulsion, VSEPR 59—62
Lone pair-bonding pair (lp-bp) repulsion, VSEPR 60—63
Lone pair-lone pair (lp-lp) repulsion, VSEPR 60—63
Lone pairs 51
Lone pairs, structures containing 61
Low spin complexes 347 348 351 367
Low symmetry groups 82—84
Low symmetry molecules 84
Lowest Unoccupied Molecular Orbital (LUMO) 127 137
Lowry, T.M. 167
LS coupling 382 391
Luminescence, and LED 227
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



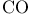
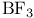
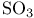 ,
, 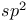
 transition metal ions, LFSE
transition metal ions, LFSE 
 bond angle
bond angle  helix
helix  in mine tailings
in mine tailings  and CR
and CR  and
and 
![$[Co(tren)(sal)]^+$](/math_tex/565f1a38dc73cf91922f4f04dcfeaa0782.gif)
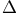
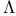
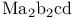
![$\mathrm{[PtCl_2(NH_3)_2]}$](/math_tex/cf8ec176e2dfb7c906e31ae8f11e9e4d82.gif)
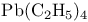
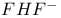
 bonding
bonding 
 donor and spectra
donor and spectra