|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Miessler G., Tarr D.A. — Inorganic Chemistry |
|
|
 |
| Предметный указатель |
Square-planar complexes, sixteen-electron 465 466
Square-pyramidal complexes 328
Stability constants of complexes 337 338
Stability of nuclei, curve 7 8
Stannane, structure 271
State, defined 385
Steam reforming 550
Stereochemistry of reactions 428—435
Stereochemistry of reactions of acid aquation 429—431
Stereochemistry of reactions of base substitution 429—431
Stereoisomerism 310
Steric effects 199 200
Steric number $\mathrm{(SN)} 57
Stibine $\mathrm{(SbH_3)} bond angle 66
Stibines $\mathrm{(SbR_3)} 276
Stone, F.G.A. 551
Strong and weak acids and solvent 202
Strong field limit 391
Strong ligand field 347
Strong-field ligands 346
Structural isomers 310
Subatomic particles 17
Sublimation 138
Sublimation, Born — Haber cycle 220
Substituted pyridines 199
Substitution mechanisms, classification 415
Substitution reactions 424
Substitution reactions in trans complexes 430 431
Substitution reactions of ![$\mathrm{[Co(NH_3)_(H_2O)]^{3+}}$](/math_tex/31c80693f1083d647c7fe6dbf4e185f782.gif) 424 424
Substitution reactions of cis complexes 432 433
Substitution reactions of square-planar complexes 434 435
Substitution reactions on ![$\mathrm{[Co(en)_2(H_2O)X]^{n+}}$](/math_tex/bc5f842e8361168f68b0662ff1d4ecde82.gif) , rates 432 , rates 432
Substitution reactions, effect of cis-ligands on rates 425
Substitution reactions, effect of entering group on rates 425
Substitution, ligand 521 522
Substitutions in crystals 232
Sulfide minerals 10
Sulfite oxidase 595
Sulfur dioxide, from coal burning 630
Sulfur oxides 284 285
Sulfur oxides and acid rain 630
Sulfur oxides from smelting 630
Sulfur oxides, reactions 283
Sulfur trioxide, dipole moment 68
Sulfur trioxide, geometry 58
Sulfur, allotropes 282
Sulfur, environmental 629
Sulfur, properties 279
Sulfur, sources 279
Sulfuric acid 203
Sulfuric acid, dissociation 168 169 197 201
Sulfuric acid, properties 285
Sulfuric acid, synthesis 283
Superacids 203
Superconductivity 228—230
Superconductivity, BCS theory 229
Superconductivity, high-temperature 230
Superconductivity, low-temperature 228
Superconductivity, Type I 228
Superconductivity, Type II 229 230
Superoxide dismutase 595 608—611
Superoxide dismutase, reactions 610
Superoxide dismutase, structure, active site 609
Superoxide ion ( ) 128 281 609 ) 128 281 609
Symmetric stretch of 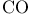 504 504
Symmetry in architecture 77
Symmetry in art 77
Symmetry in nature 77
Symmetry labels for configurations 399
Symmetry of molecular motions of water 106
Symmetry, applications 102 104 106—110
Symmetry, elements 76
Symmetry, elements and operations, table of 81
Symmetry, operations 76
Symmetry, operations and characters for ammonia 92
Symmetry, operations and characters for cis-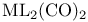 108 109 108 109
Symmetry, operations and characters for water 95—97
Symmetry, operations, matrix representation 94
Symmetry-adapted linear combinations (SALCs) 140
Synthesis gas (syn gas) 550
Talc 235
Tanabe — Sugano diagrams 390 393—395 401
Tanabe — Sugano diagrams, high spin and low spin 395
Tanabe — Sugano diagrams, strong field and weak field 395
Tellurium 279 280
Temperature dependence of equilibrium constants 192
Temperature dependence of resistivity in semiconductors, metals 228
Temperature, critical, magnetic 228
Tempering of metals 214
Template reactions 448
Template reactions, Schiff base 449
Term, defined 385
Tetraefhyllead  262 627 262 627
Tetraefhyllead  , antiknock compound 627 , antiknock compound 627
Tetragonal distortions of the octahedron 330
Tetrahedral complexes 3 327
Tetrahedral complexes and electronic structure 342
Tetrahedral complexes,  bonding 360 361 bonding 360 361
Tetrahedral complexes,  bonding 360 bonding 360
Tetrahedral complexes, absorption spectra 390
Tetrahedral complexes, molecular orbitals 360 361
Tetrahedral geometry, VSEPR 60 62—65
Tetrahedral holes in crystal lattice 210 213
Tetramminecopper(II) 299
Thallium 260
Thermodynamic measurements of acid-base interaction 193
Thermodynamics of complex formation 337 338
Thermodynamics of crystal formation 220
Thiocarbonyl (CS) 475
Thiocyanate, 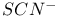 , structure 54 , structure 54
Thionitrosyl (NS) 477
Thixotropic clays 236
Thomson, J.J., e/m ratio of electron 17
Three-center two-electron bond in 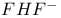 143 143
Three-center two-electron bond in 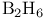 256—258 256—258
Tin allotropes 266
Titration 202
Tobacco mosaic virus, symmetry 77 78
Tolman, C.A. 523
Total pairing energy 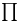 349 349
Totally symmetric representation 100
trans effect 437—440
trans effect,  bonding effects 440 bonding effects 440
trans effect,  bonding effects 439 bonding effects 439
| trans influence 439
trans-![$\mathrm{[CoX_2(trien)]^+}$](/math_tex/afc862274ad281baa594b57c1fcf79b382.gif) , chiral structures 318 , chiral structures 318
Transferrin 595 604
Transformation matrix 95 96 99
Transition metals 17
Transitions, electronic, H-atom 19 21
Translational representations 107
Translational symmetry 101
Tricapped trigonal prismatic geometry 333
Tricarbide ion, structure 268
Trifluoromethanesulfonic acid (triflic acid) 203
Trigonal antiprismatic geometry 330
Trigonal bipyramidal complexes 328
Trigonal bipyramidal geometry, VSEPR 58 60—63
Trigonal prismatic geometry 330 331
Triiodide ion,  , structure and shape 62 , structure and shape 62
Tritium, production 247
Tritium, uses 247
Tryptophan dioxygenase 595
Twist mechanisms for isomerization 434
Tyrosinase 595
Ultraviolet spectra and acid-base reactions 192
Ungerade, orbital symmetry 124
Unimolecular mechanism 522
Unit cell 207 209 210 212—214
Uranyl  , acidity 198 , acidity 198
Usanovieh, acid-base definition 166
V(IV) compounds, anticancer agents 623
Valence band in solids 223
Valence bond theory 342 344
Valence bond theory, coordination compounds 304
valence electrons 52
Valence orbital potential energies 134 135
Valence shell electron pair repulsion theory (VSEPR) 57 58 60—63
Valentine, J.S. 635
van Vleck, J.H. 304 344
Vanadyl ion  , acidity 198 , acidity 198
Vaska’s complex 466
Verkade, J.G. 161
Vibrational modes 108
Vibrational modes of water 105 106
Vibrational modes, IR-active 107 109
Vibrational modes, representations 107 109
Vibrational spectra 76 103 104 106 108—110
Vibronic coupling 390
Visible spectra and acid-base reactions 192
Vitamin 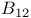 coenzyme 13 458 459 602—604 coenzyme 13 458 459 602—604
Volatron, E 162
VS and band structure 231
VSEPR 159
VSEPR and multiple bonds 62 63
VSEPR, 5-coordinate molecules 58
VSEPR, 7-coordinate molecules 58
VSEPR, distorted T geometry 61
VSEPR, square antiprism 59
Wacker (Smidt) process 541
Wade, K. 575
Wade’s rules, boranes 575
Wade’s rules, metallaboranes 579—582
Wade’s rules, metallacarboranes 579—582
Wang, J.H. 598
Water exchange rate and ion radius 422
Water exchange rate and LFSE 421
Water exchange rate and oxidation state 421
Water gas reaction 549
Water gas shift reaction, homogeneous catalysis 551
Water, boiling point 69
Water, bond angle 66
Water, dipole moment 68
Water, electron repulsion in 60
Water, electron-dot structure 52
Water, hybrid orbitals 158
Water, hydrogen bonding 69 71
Water, symmetry 103 104 106 148
Water, symmetry of molecular motions 103 104 106
Water, symmetry operations 96 97
Water, vibrational modes 105 106
Water, vibrational motions 103 104 106
Water, VSEPR and bond angle 60
Wave equation, H-atom 21—23
Wave equation, particle in a box 23 24
Wave function 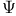 21 22 116 21 22 116
Wave function 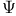 , properties 22 23 , properties 22 23
Wave function 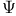 , real and complex 27 , real and complex 27
Wayland, B.B. 189
Weak ligand field 347
Weak-field ligands 346
Wells, A.E 14 237
Werner, A. 12 300 301 567
Werner, A., theory of coordination compounds 300—302
Wilkinson, F 450
Wilkinson, G. 14 450 551 636
Wilkinson’s catalyst, 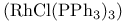 466 542 466 542
Work-hardening of metals 213
Wulfsberg, G. 14
Wurtzite, ZnS, structure 216
X-ray crystallography, for isomer identification 322
Xanthine oxidase 595
Xe as ligand 294
Xe, oxygen compounds 295
Xenon 291—195
Xenon fluorides as fluorinating agents 295
Xenon fluorides, structures 293 294
Xenon hexafluoride, structure 294
Xenon, compounds 292—294
Xenon, reactions 295
Z*, effective nuclear charge 38 40 41
Z*, effective nuclear charge and electronegativity 64
Zeise, W.C 457
Zeise’s salt, ![$\mathrm{K[Pt(C_2H_4)Cl_3]\dot H_2O}$](/math_tex/e155c9bb98d7562a0c9d47ea995ff49a82.gif) 457 482 483 457 482 483
Zeolites 236
Zeolites, cat litter 237
Zeolites, oil absorbent 237
Ziegler — Natta polymerization 549
Ziegler — Natta polymerization, Cossee — Arlman Mechanism 549
Ziegler — Natta polymerization, metallacyclobutane intermediate 548
Ziegler, K. 548
Ziegler, K., polymerization catalyst 12
Zinc blende structure 214 216
Zinc enzymes 606—608
Zintl ions 585
ZnS, radius ratio 219
[Mabcdef] isomers 313
“Cage” organometallic complexes 495
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



![$\mathrm{[Co(NH_3)_(H_2O)]^{3+}}$](/math_tex/31c80693f1083d647c7fe6dbf4e185f782.gif)
![$\mathrm{[Co(en)_2(H_2O)X]^{n+}}$](/math_tex/bc5f842e8361168f68b0662ff1d4ecde82.gif) , rates
, rates  )
) 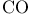
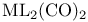

 bonding
bonding  bonding
bonding 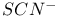 , structure
, structure 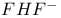
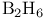
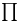
![$\mathrm{[CoX_2(trien)]^+}$](/math_tex/afc862274ad281baa594b57c1fcf79b382.gif) , chiral structures
, chiral structures  , structure and shape
, structure and shape  , acidity
, acidity  , acidity
, acidity 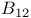 coenzyme
coenzyme 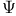
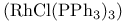
![$\mathrm{K[Pt(C_2H_4)Cl_3]\dot H_2O}$](/math_tex/e155c9bb98d7562a0c9d47ea995ff49a82.gif)