|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Miessler G., Tarr D.A. — Inorganic Chemistry |
|
|
 |
| Предметный указатель |
Buckminsterfullerene, 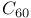 4 265 4 265
Buckminsterfullerene, 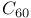 , as ligand 493—495 , as ligand 493—495
Buckminsterfullerene, 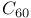 , structure 265 , structure 265
Buckyball, 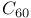 265 265
Burdett, J.K. 162
Burns, G. 237
Butanal synthesis from propene 538
C — O, stretching modes 108—110 468—471 504 506
Calcite structure 218
Calorimetric methods 192
Cannizzaro, S. 16
Capped octahedral geometry 331
Capped square antiprismatic structure 333
Capped trigonal prismatic geometry 331
Carbaboranes 577—582
Carbene (alkylidene) complexes 496 498—501
Carbene (alkylidene) complexes and heteroatoms 498
Carbene (alkylidene) complexes,  bonding 499 bonding 499
Carbene (alkylidene) complexes, 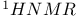 499 499
Carbene (alkylidene) complexes, Fischer-type 498
Carbene (alkylidene) complexes, methoxycarbene 498
Carbene (alkylidene) complexes, nomenclature 498
Carbene (alkylidene) complexes, Schrock-type 498
Carbide clusters 455 456 584 587 588
Carbide clusters, bonding 588
Carbide, structure 268
Carbon 261
Carbon 13 NMR of organometallic complexes 507 508
Carbon 5-coordinate 267
Carbon dioxide, electron-dot diagram 57
Carbon dioxide, geometry 57
Carbon dioxide, hybrid orbitals 158
Carbon dioxide, molecular orbitals 143—147
Carbon dioxide, properties 268
Carbon dioxide, vibrational levels and greenhouse effect 268
Carbon monoxide (CO) 267
Carbon monoxide (CO), infrared spectrum 470
Carbon monoxide (CO), molecular orbitals and photoelectron spectrum 136 138
Carbon monoxide (CO), stretching modes 108 109
Carbon monoxide (CO), symmetry 89
Carbon, 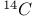 9 9
Carbon, isotopes 7 262
Carbon-centered clusters 456 577 588
Carbon-nitrogen cycle 7
Carbonate 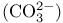 , electron-dot structure and resonance 52 , electron-dot structure and resonance 52
Carbonate 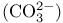 , structure and dipole moment 68 , structure and dipole moment 68
Carbonic anhydrase 595 607
Carbonic anhydrase, mechanism 608
Carbonyl (CO) complexes 467—474
Carbonyl (CO) complexes, binary 472—474
Carbonyl (CO) complexes, bonding 467—474
Carbonyl (CO) complexes, bridging 470—472
Carbonyl (CO) complexes, C — O distance 469
Carbonyl (CO) complexes, clusters 582 583
Carbonyl (CO) complexes, hydride complexes 477
Carbonyl (CO) complexes, IR spectra 468—471 503—507
Carbonyl (CO) complexes, main group parallels 556
Carbonyl (CO) complexes, synthesis of binary 473 474
Carbonyl insertion reactions, mechanism 528—532
Carbonyl stretching vibrations, cis- and trans-dicarbonyl square planar complexes 108
Carbonyl stretching vibrations, IR spectra 506
Carboranes 259 577—582
Carboranes, analogues of ferrocene 582
Carboranes, classification 578
Carborundum, properties 271
Carboxypeptidase, mechanism 595 606 607
Carbyne (afkylidyne) complexes 496—498 501 502
Carbyne (afkylidyne) complexes, bonding in 501 502
Carbyne (afkylidyne) complexes, nomenclature 498
Carbyne (afkylidyne) complexes, synthesis 534
Cartesian coordinates 28
Cat litter 236
Catalases 595 600
Catalysts, organometallic 534—551
Catalytic converters 627
Catalytic deuteration 535
Cations, acidity 197—199
Cativa process, acetic acid synthesis 540
Ceruloplasmih 595 608 609
Cesium chloride structure 214
CFSE (crystal field stabilization energy) 345
Chalcogens (Group 16) 17 279
Chalcophiles 10
Character of a matrix 96
Character of irreducible representations, properties 98
Character of molecular motions 104
Character tables 97 99—101 681—690
Character tables for octahedral complexes 346
Character tables, features 99—101
Character tables, notation 99—101
Characterization of organometallic complexes 509 510
Characters, for cis-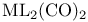 108 108
Charge transfer spectra 407
Charge transfer spectra and acid-base behavior 178
Charge transfer to ligand (CTTL) 408
Charge transfer to metal (CTTM) 407 408
Chatt, J. 182
Chauvin mechanism, olefin metathesis 544
Chelate rings 307
Chelate rings, conformations 318
Chelate rings, isomers 315
Chelates 304
Chelating ligands 304
Chemical properties and the periodic table 244
Chemical properties and the periodic table, diagonal similarities 245
Chemical properties and the periodic table, first row anomaly 245
Chemical properties and the periodic table, hydrogen 248
CHFClBr, symmetry 84 85
Chiral complexes, assigning handedness 316
Chiral isomers 316
Chiral rings, labeling 317
Chirality 102 103 311 313
Chirality, octahedral molecules 311
Chirality, tetrahedral molecules 311
Chlorin 600
Chlorine bleach 286 287
Chlorine, isolation 285
Chlorofluorocarbons, and ozone layer 632
Chlorophyll a 13
Chlorophylls 255 597 600
Chothia, C 598
Chromate 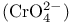 , acidity 198 199 , acidity 198 199
Chrysotile 236
Circular dichroism (CD), and chiral molecules 319 323
Cisplatin, antitumor agent 311 618
Cisplatin-DNA complex, structure 621
Class (a) metal ions 182
Class (b) metal ions 182
Class, group theory 98 100
Classes, of crystals 208
Classification, of molecule’s point group 82
Clathrates 71
Clathrates of noble gases 292
Close-packed structures 210—213
Closo borane definition 574
Cluster compounds 454 455 572—589
Cluster compounds, carbide 587 588
Cluster compounds, main group 572—579 585—587
Cluster compounds, metallaboranes and metallacarboranes 579—582
Cluster compounds, transition metal 582—585
Cluster structures, classification 577
CNCT (fulminate), VSEPR and structure 54 55
CO (carbon monoxide) 267
CO (carbon monoxide), infrared spectrum 470
CO (carbon monoxide), molecular orbitals and photoelectron spectrum 136 138
CO (carbon monoxide), stretching modes 108 109
CO (carbon monoxide), symmetry 89
CO (carbonyl) complexes 467—474
CO (carbonyl) complexes, binary 471 472
CO (carbonyl) complexes, bonding 467—474
| CO (carbonyl) complexes, bridging 470—472
CO (carbonyl) complexes, C — O distance 469
CO (carbonyl) complexes, clusters 582 583
CO (carbonyl) complexes, hydride complexes 477
CO (carbonyl) complexes, IR spectra 468—471 504 506
CO (carbonyl) complexes, main group parallels 556
CO (carbonyl) complexes, synthesis of binary 473
CO dissociation 474 521
Cobalamin 602
Cobalamin, catalysis 603
Cobaltocene 489 490
Coenzyme  13 13
Coenzyme  , vitamin 602—604 , vitamin 602—604
Coinage metals 17
Collman, J.P. 527 551
Collman’s reagent 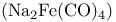 527 527
Colors, complementary 380
Colors, coordination compounds 379
Colors, gemstones 379
Complex ions 299 302
Conditions for high and low oxidation numbers 445
Conduction band in solids 223
Conductivity, and metallic character 241
Conductivity, diamond 213
Conductivity, insulators 223 224
Conductivity, metals 213 223 224
Conductivity, semiconductors 223 224
Conductivity, temperature dependence 224 228
Conductor, band structure 223 224
Cone angle, ligand 523
Configurational isomers 310
Conformations, ligand ring 318 319
Conjugate acids and bases 167
Conjugate base mechanism 426 427
Constant electron density surfaces 33
Constitutional isomers 310
Cooke, M.P. 527
Cooper pairs in superconductors 229
Coordinate covalent bond 299
Coordinate system for octahedral orbitals 353
Coordinate system for square planar orbitals 356
Coordinate system for tetrahedral orbitals 361
Coordinate system, spherical 28
Coordination compounds 299
Coordination compounds, acid-base definition 171
Coordination compounds, crystal field theory 304
Coordination compounds, defined 299
Coordination compounds, history 299—302 304
Coordination compounds, isomerism 309—313 315 316 318—320 322 323
Coordination compounds, ligand field theory 304
Coordination compounds, nomenclature 299—302 304 305 307 308
Coordination compounds, valence bond theory 304
Coordination geometry 2
Coordination isomers 309 310 320
Coordination number (CN) 2 323—333
Coordination number (CN) and electronic structure 342
Coordination number (CN) in solids 209 210 212 213 215 217—219
Coordination number (CN), CN 1, 2, and 3 323 326 327
Coordination number (CN), CN 4 327
Coordination number (CN), CN 5 328
Coordination number (CN), CN 6 329—331
Coordination number (CN), CN 7 331
Coordination number (CN), CN 8 332
Coordination number (CN), CN larger than 8 333
Coordination sphere 302
Copper enzymes 608
Correlation diagram 132 133 390—392
Correlation diagram for homonuclear diatomic molecules 132 133
Correlation diagram for octahedral transition metal complexes 391 392
Cosmic rays 9
Cossee — Arlman mechanism 533 549
Cotton effect, in ORD and CD 323 324
Cotton, F.A. 14 110 162 636
Coulomb energy 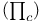 , electron repulsion 35 36 , electron repulsion 35 36
Coulomb energy 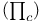 , transition metal complexes 347—349 351 , transition metal complexes 347—349 351
Counting electrons, 18-electron rule 460—463
Counting electrons, 18-electron rule, donor pair method 460
Counting electrons, 18-electron rule, neutral ligand method 460
Covalent character, and acid-base reactions 181 182
Covalent radii 44 45
Cowan, J.A. 635
Cox, P.A. 14 237
Cr(II), Jahn — Teller effect 372
Creation of the universe 5
Creswick, R.J. 237
Critical temperature 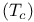 for superconductivity 228 for superconductivity 228
Crown ether, alkali metal complexes 251
Cryptand, alkali metal complexes 251
Crystal field splitting 344
Crystal field stabilization energy (CFSE) 345
Crystal field theory 12 304 342 344
Crystal radii 46 47
Crystal structures, binary compounds 214—217
Crystal structures, body centered cubic 210
Crystal structures, close-packed 210—213
Crystal structures, CsCl 215
Crystal structures, cubic close packed 210 212
Crystal structures, diamond 213
Crystal structures, face centered cubic 209 210 212 213
Crystal structures, fluorite 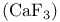 216 216
Crystal structures, hexagonal close packed 210 212
Crystal structures, NaCl 215
Crystal structures, NiAs 217
Crystal structures, primitive cubic 209
Crystal structures, rutile 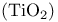 217 217
Crystal structures, wurtzite 215 216
Crystal structures, zinc blende 215
Crystallization, fractional, for separation of isomers 322
CS (thiocarbonyl) 475
CsCl structure 215
CSe (selenocarbonyl) 475
Cu(II), Jahn-Teller effect 372
Cubic close packing (ccp) 210—213
Cubic geometry and VSEPR 59
Cyanate, 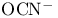 , structure 54 , structure 54
Cyanide 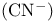 as ligand 353 354 456 475 as ligand 353 354 456 475
Cyanide 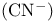 , molecular orbitals 353 354 , molecular orbitals 353 354
Cyanogen, NCCN, as pseudohalogen 290
Cyclic  systems 480—482 485 486 489—491 systems 480—482 485 486 489—491
cyclo-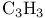 , as ligand 480—482 , as ligand 480—482
Cyclobutadiene, as ligand 481
Cyclometallation reactions 525
Cyclooctadiene complexes 484
Cyclopentadienyl (Cp) 485
Cyclopentadienyl (Cp) as ligand 458—460 485 486 489—491
Cyclopentadienyl (Cp), complexes 485 486
Cytochromes 595 597 599 600
d Orbital interactions 569
d Orbitals in octahedral complexes, energies 364 366
Dalton, John 15 16
Davies, N.R. 182
de Broglie, L., equation 19
Degenerate orbitals 36 127 133
Degrees of freedom, molecular motion 103 104
Delta 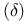 bond 1 bond 1
Delta 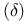 orbitals, from d orbitals 120 orbitals, from d orbitals 120
Democritus 15
Denitrification 612
Density of states, N(E), in solids 223 224
Deuterium 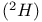 6 247 6 247
Dextrorotatory 102
Diamagnetic compounds, magnetic susceptibility 125 339
Diamminedichloroplatinum(II), ![$\mathrm{[PtCl_2(NH_3)_2]}$](/math_tex/cf8ec176e2dfb7c906e31ae8f11e9e4d82.gif) , cis and trans isomers 308 , cis and trans isomers 308
diamond 214 263 364
Diastereomers 310
Diatomaceous earth 269
Diazene 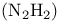 274 275 274 275
Diborane 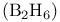 , bonding 256—258 , bonding 256—258
Dicarbide ion, structure 268
Dickerson, R.E. 598
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



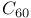
 bonding
bonding 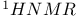
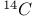
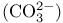 , electron-dot structure and resonance
, electron-dot structure and resonance 
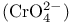 , acidity
, acidity 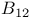
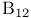 , vitamin
, vitamin 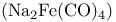
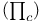 , electron repulsion
, electron repulsion 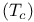 for superconductivity
for superconductivity 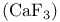
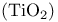
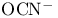 , structure
, structure 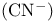 as ligand
as ligand 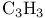 , as ligand
, as ligand 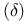 bond
bond 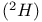
![$\mathrm{[PtCl_2(NH_3)_2]}$](/math_tex/cf8ec176e2dfb7c906e31ae8f11e9e4d82.gif) , cis and trans isomers
, cis and trans isomers 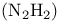
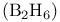 , bonding
, bonding