|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Metzger J.V. — Thiazole and Its derivatives (Part 2) |
|
|
 |
| Предметный указатель |
Staphylococcus aureus, treatment of 440
Steric decompression, in alkylation of  -4-thiazoline-2-thione 391 -4-thiazoline-2-thione 391
Steric effect and HSAB pattern 63
Steric effect in coupling with diazonium 76
Steric effect in nitrosation 68
Steric effect in rearrangement 102 130
Steric effect in relation with, 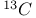 spectra 384—385 spectra 384—385
Steric effect in relation with, X-Ray of  -4-thiazoline-2-thione 385 13 -4-thiazoline-2-thione 385 13
Steric effect on ambident reactivity of  -4-thiazoline-2-thione anion 394 -4-thiazoline-2-thione anion 394
Steric effect on aminothiazoles alkylations 39
Steric effect on protomerism of  -4-thiazoline-2-thione 380 -4-thiazoline-2-thione 380
Steric effect on rates of quaternaiisation 32
Steric effect on S/N rearrangement in thiazolyl-2-thioethers 406
Steric effect on self association of  -4-thiazoline-2-thione 384 -4-thiazoline-2-thione 384
Steric effect, in alkylations 69
Steric inhibitor 135
Sterility in male rats 149 153
Stimulant of central nervous system 147
Streptococcus 152
Structure determinations, of alkylated amino and iminothiazoles, by chemical methods 37
Structure determinations, of alkylated amino and iminothiazoles, by spectroscopic methods 38
Structure determinations, of aminothiazoles, by X-ray 29
Structure determinations, of azo thiazoles by X-ray 108
Structure determinations, of complexes 120
Structure determinations, of hydrazinothiazoles 100
Structure determinations, of Schiff bases 99
Structure determinations, of sulfonylthiazoles 75
Structure determinations, of tautomeric isomers 113
Styryl derivatives, from alkyl-acetamidothiazoles 46
Sublimation, of 2-aminothiazoles 30
Substituent effects on self-association of  -4-thiazoline-2-thione 377 -4-thiazoline-2-thione 377
Substituent effects, in relation with, HMO calculation protomeric  -4-thiazoline-2-thione 377 378 -4-thiazoline-2-thione 377 378
Substituent effects, of amino and acetanrido 91
Substituent effects, of thiazole as substituent 71
Substituent effects, on acvlations 48
Substituent effects, on alkylations 112
Substituent effects, on ambident reactivity 36 40 39 63
Substituent effects, on complexes 122
Substituent effects, on cydizations 62 63
Substituent effects, on diazotization 67 76
Substituent effects, on mass spectra 28
Substituent effects, on nitration 74 85
Substituent effects, on NMR spectra 25
Substituent effects, on nucleophilic attack 18
Substituent effects, on pKa 19
Substituent effects, on protomeric equilibrium 2 19 20 97
Substituent effects, on protomerism in  -2-thiazoline-5-ones 431 -2-thiazoline-5-ones 431
Substituent effects, on rates 32
Substituent effects, on reaction of halogenothiazoles and MeOH 409
Substituent effects, on reactivity with aldehydes 45
Substituent effects, on rearrangement 82 95 102 114
Substituent effects, on UV spectra 22
Substitution reactions on aromatic derivatives 56
Substitution reactions, by aminothiazoles towards 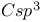 electrophilic centers 32 electrophilic centers 32
Succinate dehydrogenase 135
Sudoxicam 138 145
Sugar, with 2-hydrazinothiazoles 101
Sulfamic acid derivatives see Thiazolyl sulfamic acids
Sulfate anion 121
Sulfathiazoles, methylation with 37
Sulfathiazoles, UV spectra of 20 see
Sulfenarnido thiazoles, preparation of, from  -4-thiazoline-2-thione 411 -4-thiazoline-2-thione 411
Sulfenarnido thiazoles, preparation of, from thiazolyl disulfides 411
Sulfenarnido thiazoles, reaction of, with amines 411
Sulfenyl chloride (p-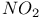 -phenyl), reaction with, -phenyl), reaction with,  -4-thiazoline-2-one 403 -4-thiazoline-2-one 403
Sulfenyl chlorides see Arylsulfenyl chlorides
Sulfonamides 114 414
Sulfonamidothiazoles 114 299—316
Sulfonamidothiazoles, biological activity of 114
Sulfonamidothiazoles, cleavage of 117
Sulfonamidothiazoles, lR spectra of 116
Sulfonamidothiazoles, methylation of 116
Sulfonamidothiazoles, polymorphism of 116
Sulfonamidothiazoles, reactivity of 116
Sulfonamidothiazoles, synthesis of 115
Sulfonamidothiazoles, UV spectra of 116
Sulfonation 75
Sulfonation of  -4-thiazoline-2-one, 4-position 403 -4-thiazoline-2-one, 4-position 403
Sulfonation of  -4-thiazoline-2-one, 5-position 402 -4-thiazoline-2-one, 5-position 402
Sulfonation of 2-aminothiazoles 75
Sulfonation of 2-azidothiazoles 113
Sulfonation of 2-substituted thiazoles 413
Sulfonation of 5-thiazolyl oxide 436
Sulfonation of pyridines 75
Sulfonation of thiazole 413
Sulfonic acid, sulfamic acid rearrangement 70
Sulfonimidothiazolines 317 318
Sulfonyl halides, with 2-aminothiazoles 69
Sulfur (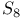 ), in synthesis of, ), in synthesis of,  -4-thiazoline-2-thione 373 -4-thiazoline-2-thione 373
Sulfur compounds 69
Sulfuric acid in bromination 77
Sulfuric acid in nitrations 72
Sulfuric acid, dealkylation by 39
Sulfuric acid, deuterated 92
Sulfuric acid, diazotization with 66
Sulfuric acid, rearrangements in 73 113
Sulfuric acid, with alcohols 38 47 80 90
Sulfuryl chloride 432
Symbiotic behavior see HSAB theory
Symmetry, 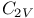 and and 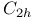 120 120
Synthetic fibers 154
TAC, 3-(2-thiazolylazo)-p-cresol 107 109
TAM, 1(2-thiazolylazo)-2-hydroxy-4-dimethylaminobenzene 107 109 158
TAN (or Htan), 1-(2-thiazolylazo)-2-naphtol 107 109 154 160
TAN 3,6-S 1-(2-thiazolylazo)-3,6-disulfo-2-naphtol 107 109
TAT, 4-(2-thiazolylazo)-thymol 107 109
Tautomerism, amide-imide 116. See also Protomeric equilibrium
Tetmnychus urticae 442
Tetrahedral structure 120
Tetrazole, 6-phenylthiazolo [2,3]- 414
Tetrazole, derivatives 113
Textiles 170
THAN, 1-(2-thiazolylazo)-2-hydroxy-3-naphtoic acid 107 109
Therapeutic activity 152
Thermal induction 122
Thiadiazine derivatives 102
Thiadiazole derivatives 95 96
Thiamine, biosynthesis inhibition 152
Thiamine, model for thiamine action 375
Thiazole blue 103
Thiazole, 2,5-dimethyl, sulfonation 413
Thiazole, 2-azido-4-phenyl-5 sulfonic acid, preparation 414
Thiazole, 2-substituted, sulfonation 413
Thiazole, 2-substituted, to 5-chlorosulfonyl derivatives 414
Thiazole, 2-unsubstituted, from  -4-thiazoline-2-ones 402 -4-thiazoline-2-ones 402
Thiazole, 2-unsubstituted, from  -4-thiazoline-2-thiones 393 -4-thiazoline-2-thiones 393
Thiazole, 2-unsubstituted, from 2-thiazolesulfinic acid 413
Thiazole, 2-unsubstituted, from 2-thiazolesulfonic acid 414
Thiazole, 2-unsubstituted, from 2-thiazolyldisulfide 412
Thiazole, 2-unsubstituted, from 2-thiazolylthioether 405
Thiazole, 5-acetylthio, preparation 417 428
Thiazole, 5-acetylthio, to 5-unsubstituted thiazole 417
Thiazole, 5-bromo, reaction with mercaptans 418
Thiazole, 5-ethylthio 418
Thiazole, 5-thiocyanato, oxidation with nitric acid 418
Thiazole, 5-thiocyanato, preparation 417
Thiazole, 5-thiocyanato, table of compounds 496 497
Thiazole, 5-thiocyanato, to  -2-thiazoline-5-thione 416 -2-thiazoline-5-thione 416
Thiazole, 5-unsubstituted, from 5-acetylthiazole 417
Thiazole, sulfonamide, from  -4-thiazoline-2-thione 414 -4-thiazoline-2-thione 414
Thiazole, sulfonamide, from chlorosulfonylthiazoles 414
Thiazole, sulfonation 413
Thiazole-4-sulfonic acids, preparation of, from 2,5-dibromothiazole 413
Thiazole-5-sulfonic acids, preparation of 413
Thiazole-5-sulfonic acids, table of compounds 502
Thiazolium salts, hydratation of 84
Thiazolium salts, oxidation in basic media 375—377
Thiazolium salts, proton abstraction in 375 376
Thiazolium salts, reaction with Sg in basic media 375—377
Thiazolooxazines 426
| Thiazolopyridazines 426
Thiazolotetrazole-azidothiazole 113
Thiazolyl area 92
Thiazolyl area with acetic anhydride 93
Thiazolyl area with sodium acetate 93
Thiazolyl area, alkylation of 93
Thiazolyl area, cyclization of 37 56
Thiazolyl area, formation of 56 58 92
Thiazolyl area, infrared data of 93
Thiazolyl area, NMR data of 93
Thiazolyl area, UV data of 93
Thiazolyl carbamates 96 97 241—242
Thiazolyl disulfides, as vulcanization accelerators 412
Thiazolyl disulfides, oxidation of 411
Thiazolyl disulfides, preparation of 412
Thiazolyl disulfides, preparation of, from  -4-thiazoline-2-thione and -4-thiazoline-2-thione and 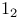 393 412 393 412
Thiazolyl disulfides, preparation of, from  -4-thiazoline-2-thione and -4-thiazoline-2-thione and 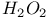 393 393
Thiazolyl disulfides, preparation of, from  -4-thiazoline-2-thione and ammonium persulfate 393 412 -4-thiazoline-2-thione and ammonium persulfate 393 412
Thiazolyl disulfides, transformation in,  -4-thiazoline-2-thiones 412 -4-thiazoline-2-thiones 412
Thiazolyl disulfides, transformation in, sulfenamido-thiazoles 411 412
Thiazolyl disulfides, unsymmetrical, preparation of, from sulfenyl chloride 412
Thiazolyl disulfides, uses in photographic processes 412
Thiazolyl guanidines 97 98 219
Thiazolyl guanidines, complexes of 98
Thiazolyl sulfamic acids, by sulfonation 75
Thiazolyl sulfamic acids, rearrangement of sulfonic acid 70
Thiazolyl sulfamic acids, rearrangement to sulfonic acid 75
Thiazolyl thiourea 92 243—246
Thiazolyl thiourea with benzyl bromide 95
Thiazolyl thiourea, chelates with 95
Thiazolyl thiourea, desulfurization of 95
Thiazolyl thiourea, formation of 59 92
Thiazolyl thiourea, formation of tertiary 125
Thiazolyl thiourea, infrared spectra of 94
Thiazolyl thiourea, mass spectra of 94
Thiazolyl thiourea, rearrangement of 95
Thiazolyl thiourea, UV spectra of 94
Thiazolyl-2-thioglycolic acid 407
Thiazolyl-4-phosphoric esters 423
Thiazolylamidines 98
Thiazolylamidines, acid hydrolysis of 99
Thiazolylamidines, reduction of 99
Thiazolylazo see Azothiazoles
Thiazolylcholesteryl urethanes 97
Thiazolylcyanamide 92 97
Thiazolylcyanamide to guanidmo derivatives 97
Thiazolylcyanamide with hydrogen sulfide 94
Thiazolylhydrazines 248—258 267.
Thiazolylsemicaibazides 103
Thin-layer chromatography of  -4-thiazoline-2-thione 385 -4-thiazoline-2-thione 385
Thin-layer chromatography of 2-imino-4-thiazolines 124
Thin-layer chromatography of sulfathiazole 116
Thin-layer chromatography of thiazolyl amidines 99
Thin-layer chromatography of thiazolyl thioureas 94
Thin-layer chromatography, 2-amino-thiazole identification by 30
Thioacetic acid, reaction with,  -2-oxazoline-5-one 417 418 429 -2-oxazoline-5-one 417 418 429
Thioacetic acid, reaction with,  -2-thiazoline-5-one 417 418 -2-thiazoline-5-one 417 418
Thioacetic acid, reaction with, 4-chloro, 4-Me, 2-Ph- -2-thiazoline-5-one 432 -2-thiazoline-5-one 432
Thiobarbituric acid 95
Thiocarbonyl group, nucleophilicity of 95
Thiochrome 124
Thiochrome from  -4-thiazoline-2-one 402 -4-thiazoline-2-one 402
Thiocyanate, with 2-azidothiazoles 113
Thiocyanate, with 2-hydrazinothiazoles 100
Thiocyanate, with azothiazoles 108
Thiocyanate, with halogenothiazoles 79
Thiocyanation, of  -4-thiazoline-2-thione 400 -4-thiazoline-2-thione 400
Thiocyanation, of thiazolyl-2-sulfone 415
Thiophene, 2,5-diphenyl-3,4-dicarboxy-methyl, from  -2-thiazoline-4-one 426—427 -2-thiazoline-4-one 426—427
Thiourea 79 243—246
Thiourea, reaction with, 2-bromothiazole 370
Thiourea, reaction with, 2-thiazolyldiazonium salts 370
Thiourea, reaction with, 5-amino-4-methyl thiazole 417
Thiourea, substituted, from 1,3-dithiole-2-thione and amine 372 see
Thiuram 61
Thorium complexes 161
Thymoanaleptic 152
Tin complexes, reaction with  -4-thiazoline-2-thiones 394 -4-thiazoline-2-thiones 394
Tinctorial strength 154
Titanium complexes 156
Titration 154 159
Titration of aminothiazole 30
Toluene sulfonyl see Tosyl chloride
Tomatoes 136
Tosyl chloride, alkylations with 69
Toxicity 138 153
Tranquilizing activities 147
Trans isomers 120
Transition metal complexes 70 119
Transition metal complexes of  -4-thiazoline-2-thiorie 386 see -4-thiazoline-2-thiorie 386 see
Triazene derivatives 113
Triazepine derivatives 101 102
Triazine, by cyclization 65 129
Triazole derivatives 130
Trichomonacidal 141
Trichomonas vaginalis 139
Tridentate ligand 120
Triethylamine, as base catalyst 48
Trifluoroacetic acid 426
Trimethylene dibromide, with aminothiazoles 33
Triphenylmethylchloiide, with aminothiazoles 34
Triphenylphosphine, imidazole mixture as catalyst 90
Trirnethylsilyl chloride, silylation of 2-aminothiazoles 68
Triticum vulgare 134
Tuberculostatic activity 141 440 441.
Tungsten complexes 156
Ullmann synthesis 115
Unsaturated acid chlorides, acylation 49
Uranium derivatives 154
Uranyl ions 108
Urea, to thiazolyl urea 56 see
Urea, with azothiazoles 111
Urine, 2-aminothiazoles in 85
Urine, metabolite in 85
Uses, aminothiazoles derivatives 132—172
Uses, hydroxythiazole derivatives 438—442
Uses, mercaptothiazole derivatives 438—442
UV spectra 21
UV spectra and amino-imino equilibrium 19 20
UV spectra and charge transfer 21
UV spectra and potentiometric measurements 21
UV spectra and PPP calculations 21
UV spectra and solvent effects 21
UV spectra and substituent effects 22
UV spectra of  -2-thiazoline-4-one 422 -2-thiazoline-4-one 422
UV spectra of  -2-thiazoline-4-one, -2-thiazoline-4-one,  determination 423 determination 423
UV spectra of  -4-thiazoline-2-one, in relation with protomerism 387 -4-thiazoline-2-one, in relation with protomerism 387
UV spectra of  -4-thiazoline-2-one, representative data 389 -4-thiazoline-2-one, representative data 389
UV spectra of  -4-thiazoline-2-thiones 380 -4-thiazoline-2-thiones 380
UV spectra of  -4-thiazoline-2-thiones in relation with protomerism 378 -4-thiazoline-2-thiones in relation with protomerism 378
UV spectra of 2-thiazolylthioethers 404
UV spectra of alkylaminothiazoles 38
UV spectra of iminothiazolines 38
UV spectra of Schiff bases 41
UV spectra of sulfathiazoles 20
UV spectra of sulfonamidothiazoles 116
UV spectra of thiazolyl-2-oxides 409
UV spectra of thiazolyl-4-oxides 426
UV spectra of thiazolylcaibamates 97
UV spectra of thiazolylthioureas 94 96
UV spectra of thiazolylureas 93
UV spectra, representative data 22
Vanadium complexes 157 158
Vapor phase chromatography see Gas-liquid chromatography
Vaso-depressor 149
Vasodilator 150
Veterinary applications 138
Vinylchloride 438
Virus inhibitor 140. See also Antiviral agent
Vulcanization accelerators 438 440 441
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



 -4-thiazoline-2-thione
-4-thiazoline-2-thione 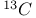 spectra
spectra 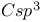 electrophilic centers
electrophilic centers 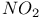 -phenyl), reaction with,
-phenyl), reaction with, 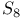 ), in synthesis of,
), in synthesis of, 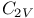 and
and 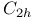
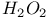
 determination
determination