|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Metzger J.V. — Thiazole and Its derivatives (Part 2) |
|
|
 |
| Предметный указатель |
2-Thiazolyl sulfide, tables of compounds, 5-nitro 454 455
2-Thiazolyl sulfide, tables of compounds, 5-nitro-2-heteroaryl 453
2-Thiazolyl sulfide, tables of compounds, 5-substituted 450
2-Thiazolyl sulfide, tables of compounds, 5-substituted benzylideneamino 451 452
2-Thiazolyl sulfide, tables of compounds, S-naphtoquinonyl derivatives 504
2-Thiazolyl sulfide, transformation in, 2-thiazolyl sulfones 405 415
2-Thiazolyl sulfide, transformation in, 2-unsubstituted thiazoles 405
2-Thiazolyl sulfide, uses of 440
2-Thiazolyl sulfinicacid, decomposition of 413
2-Thiazolyl sulfinicacid, preparation of, from  -4-thiazoline-2-thione and -4-thiazoline-2-thione and 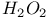 393 413 393 413
2-Thiazolyl sulfinicacid, preparation of, from 2-acetamidothiazole sulfonyl chloride 413
2-Thiazolyl sulfinicacid, table of compounds 472—473
2-Thiazolyl sulfonic acids, preparation of, from  -4-thiazoline-2-thione and -4-thiazoline-2-thione and 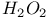 397 413 397 413
2-Thiazolyl sulfonic acids, preparation of, from  -4-thiazoline-2-thione and -4-thiazoline-2-thione and 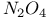 393 413 393 413
2-Thiazolyl sulfonic acids, preparation of, from direct sulfonation 413
2-Thiazolyl sulfonic acids, tables of compounds 472 473
2-Thiazolyl sulfonic acids, transformation in,  -4-thiazolme-2-one 375 -4-thiazolme-2-one 375
2-Thiazolyl sulfonic acids, transformation in, 2-N-alkylaminothiazole 414
2-Thiazolyl sulfonic acids, transformation in, 2-unsubstituted thiazole 414
2-Thiazolyl sulfonyl chloride, preparation of, from  -4-thiazoline-2-thione 414 -4-thiazoline-2-thione 414
2-Thiazolyl sulfonyl chloride, transformation to, sulfonamides 414
2-Thiazolyl sulfoxides, table of compounds 471
2-Thiazolyl-sulfones, bromination of 415
2-Thiazolyl-sulfones, nitration of 415
2-Thiazolyl-sulfones, preparation of, from 2-halothiazole 415
2-Thiazolyl-sulfones, preparation of, from 2-thiazolyl sulfide 405 415
2-Thiazolyl-sulfones, tables of compounds 472—473
2-Thiazolyl-sulfones, thiocyanation of 415
3-Allyl- -4-thiazoline-2-one 410 -4-thiazoline-2-one 410
3-Amino-2-iminothiazoline, from thiazolium salts 130
3-Amino-2-iminothiazoline, nicotinoylation reaction with 131
3-Amino-2-iminothiazoline, to, 1,3,4-oxadiazole 132
3-Amino-2-iminothiazoline, with acid chlorides 131
3-Amino-2-iminothiazoline, with carbon disulfide 131
3-Amino-2-iminothiazoline, with cyanogen bromide 131
3-Amino-2-iminothiazoline, with ethyl orthoformate 131
3-Bromo-2-butanone 85
3-Chloropropionic acid, quaternization with aminothiazoles 32
3-Ethyl-4-hydroxy- -4-oxazoline-2-thione 407 -4-oxazoline-2-thione 407
4,5-Dialkyl-2-ammothiazoles 186
4,5-Diaryl-2-aminothiazoles 193—199
4,5-Dihetaryl-2-aminothiazole 193—199
4,5-Disubstituted-2-aminothiazoles 186—192
4,5-Disubstituted-2-substituted aminothiazoles 215—218
4-Alkyl-2 aminothiazoles 172
4-Alkylidene- -2-thiazoline-5-one 427 -2-thiazoline-5-one 427
4-Alkylidene- -2-thiazoline-5-one, Friedel — Ctafts reaction on 428 -2-thiazoline-5-one, Friedel — Ctafts reaction on 428
4-Alkylidene- -2-thiazoline-5-one, Grignard reaction on 429 -2-thiazoline-5-one, Grignard reaction on 429
4-Alkylidene- -2-thiazoline-5-one, preparation of, from -2-thiazoline-5-one, preparation of, from  -2 thiazoline-5-one and enamines 432 -2 thiazoline-5-one and enamines 432
4-Alkylidene- -2-thiazoline-5-one, preparation of, from -2-thiazoline-5-one, preparation of, from  -2-thiazoline-5-one and aldehyde 432 -2-thiazoline-5-one and aldehyde 432
4-Alkylidene- -2-thiazoline-5-one, preparation of, from -2-thiazoline-5-one, preparation of, from  -2-thiazoline-5-one and ketone 432 -2-thiazoline-5-one and ketone 432
4-Alkylidene- -2-thiazoline-5-one, reaction with phenyl lithium 429 -2-thiazoline-5-one, reaction with phenyl lithium 429
4-Alkylidene- -2-thiazoline-5-one, reduction of 427 -2-thiazoline-5-one, reduction of 427
4-Aminothiazole-2,5-diphenyl, to 2,5 di-phenyl- -2-thiazoline-4-one 421 -2-thiazoline-4-one 421
4-Aminothiazoles 295 296
4-Aminothiazoles, ambident reactivity of 31
4-Aminothiazoles, with  -diketones 46 -diketones 46
4-Aminothiazoles, with benzaldehyde 47
4-Aminothiazoles, with chlorovinyl ketones 46
4-Aminothiazoles, with isocyanates 60
4-Aminothiazoles, with isothiocyanates 60
4-Aryl-2-aminothiazoles 178—182
4-Aryl-2-aminothiazoles, mercuration of 81
4-Aryl-2-aminothiazoles, nitration of 72
4-Aryl-2-aminothiazoles, reduction of 86
4-Aryl-2-aminothiazoles, Schiff bases from 99
4-Bromothiazole 426
4-Chloro-4-methyl-2-phenyl- -2-thiazoline-5-one 432 -2-thiazoline-5-one 432
4-Chloro-4-methyl-2-phenyl- -2-thiazoline-5-one from 4-methy]-2-phenyl- -2-thiazoline-5-one from 4-methy]-2-phenyl- -2-thiazoline-5-one 432 -2-thiazoline-5-one 432
4-Chloro-4-methyl-2-phenyl- -2-thiazoline-5-one, reaction with thioacetic acid 432 -2-thiazoline-5-one, reaction with thioacetic acid 432
4-Chlorophenol, hydrogen bonding, with  -4-thiazoline-2-thione 377 -4-thiazoline-2-thione 377
4-Formyl pyridine 398
4-Hetaryl-2-aminothiazoles 178—182
4-Hydroxythiazole see  -2-Thiazolirie-4-one -2-Thiazolirie-4-one
4-Mercapto-imidazoline-2-thione, from 5-amino- -4-thiazoline-2-thione 399 -4-thiazoline-2-thione 399
4-Methyl-5- -chloroethylthiazole 375 -chloroethylthiazole 375
4-Methylthiazole, preparation of, from 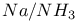 reduction of 4-methyl- reduction of 4-methyl- -4-thiazoline-2-thione 397 -4-thiazoline-2-thione 397
4-Substituted-2-aminothiazoles 172—177
4-Substituted-2-arylaminothiazoles 204—209
4-Substituted-2-hetarylaminothiazoles 210
4-Substituted-2-substituted amino-thiazoles 200—203
4-Thiazolyl oxides, NMR of 426
4-Thiazolyl oxides, preparation of 426
4-Thiazolyl oxides, tables of compounds 530
4-Thiazolyl oxides, uses of 442
4-Thiazolyl oxides, UV of 426
4-Thiazolyl sulfide, table of compounds 502—503
4-Thiazolyl-aryl sulfone, preparation of 416
4-Thiazolyl-aryl sulfone, table of compounds 503
5-Alkyl-2-aminothiazoles 183
5-Aminomethyl- -4-thiazoline-2-thione derivatives, from rearrangement of 3-aminomethyl- -4-thiazoline-2-thione derivatives, from rearrangement of 3-aminomethyl- -4-thiazoiine-2-thione 400 -4-thiazoiine-2-thione 400
5-Aminothiazoles 292—295
5-Aminothiazoles, ambident reactivity of 31
5-Aminothiazoles, by reduction of nitrothiazoles 74
5-Aminothiazoles, rearrangement 86
5-Aminothiazoles, synthesis of 16
5-Halogenothiazole, reaction with, metal sulfinate 415
5-Hydroxythiazole see  -2-Thiazoline-5-one -2-Thiazoline-5-one
5-Mercaptothiazole see  -2-Thiazoline-5-thione -2-Thiazoline-5-thione
5-Methylenethiazolidine-2-thione, preparation of, from propargyl amine and 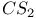 372 373 372 373
5-Nitro-2-aminothiazoles, pharmaceutical activity of 72
5-Nitro-2-aminothiazoles, rearrangement to 83
5-Substituted-2-aminothiazole 183—185
5-Substituted-2-substituted aminothiazoles 213 214
5-Thiazolyl oxides, bromination of 436
5-Thiazolyl oxides, cleavage to  -2-thiazoline-5-one 430 -2-thiazoline-5-one 430
5-Thiazolyl oxides, flavoring properties 436
5-Thiazolyl oxides, nitration of 436
5-Thiazolyl oxides, physical properties 436
5-Thiazolyl oxides, preparation of 436
5-Thiazolyl oxides, sulfonation of 436
5-Thiazolyl oxides, tables of compounds 528 529
5-Thiazolyl oxides, uses of 442
5-Thiazolyl sulfides, bis-5-thiazolyl sulfide, oxidation of 415
5-Thiazolyl sulfides, bis-5-thiazolyl sulfide, oxidation of, 5-(2-hydroxythiazolyl)phenyl sulfide case 418
5-Thiazolyl sulfides, bis-5-thiazolyl sulfide, oxidation of, general 418
5-Thiazolyl sulfides, physical properties 418
5-Thiazolyl sulfides, preparation of 417—418
5-Thiazolyl sulfides, table of compounds 493—496
5-Thiazolyl sulfides, uses of 442
5-Thiazolyl sulfmic acid, preparation of 413
5-Thiazolyl sulfmic acid, table of compounds 498—501
5-Thiazolyl sulfones, physical properties, IR 415
5-Thiazolyl sulfones, physical properties, NMR 415
5-Thiazolyl sulfones, preparation of, from 5-halogenothiazole 415
5-Thiazolyl sulfones, preparation of, from 5-thiazolyl sulfide 415 418
5-Thiazolyl sulfones, table of compounds 498—501
5-Thiazolyl sulfonyl chloride, preparation of, from 2-substituted thiazoles 414
5-Thiazolyl sulfonyl chloride, transformation to, sulfonamides 414
5-Thiazolyl sulfoxides, table of compounds 498
5-Thiazolyl thiouronium salts, preparation of 417
5-Thiazolyl thiouronium salts, to  -2-thiazoline-5-thione 416 -2-thiazoline-5-thione 416
5-Thiazolyl-acetate, preparation of 426
8-Hydroxyquinoline, with aminothiazoles 44
Acaricide 442
Acetamido-acetimido, protomeric equilibrium 91
Acetamidothiazoles 220—241
Acetamidothiazoles from acetic acid 53
Acetamidothiazoles from acetic anhydride 52 91
Acetamidothiazoles from acyl halides 48—51 90
Acetamidothiazoles to acetylimino-4 thiazolines 91
Acetamidothiazoles, alkylations 35
Acetamidothiazoles, alkylations, ambident reactivity in 37
Acetamidothiazoles, alkylations, general pattern 91
Acetamidothiazoles, alkylations, with  -chloroacetamide 33 -chloroacetamide 33
Acetamidothiazoles, aminoalkylation of 44
Acetamidothiazoles, as ligands 70 120
Acetamidothiazoles, bromination of 79
Acetamidothiazoles, by heterocyclization methods 90
Acetamidothiazoles, from esters 53
| Acetamidothiazoles, from sodium acetate 52
Acetamidothiazoles, from thiazolylureas 93
Acetamidothiazoles, IR spectra of 23
Acetamidothiazoles, mass spectra of 28
Acetamidothiazoles, mercuration of 81
Acetamidothiazoles, nitration of 72 74
Acetamidothiazoles, reduction of 92
Acetamidothiazoles, salts of 91
Acetamidothiazoles, sulfonation of 75
Acetamidothiazoles, urinary metabolite 85
Acetic acid, with 2-aminothiazole 53
Acetic acid, with ethylene oxide 38
Acetic anhydride 421 426 431
Acetic anhydride in solid state reactions 52
Acetic anhydride to 2-acetamidothiazoles 52
Acetic anhydride with 2-hydrazinothiazoles 101 106
Acetic anhydride with carbamates 16
Acetic anhydride with sulfenamidothiazoles 114
Acetic anhydride with thiazolyl ureas 93
Acetoacetamidothiazole 53
Acetophenone, by reduction 86
Acetophenone, from 4-phenyl- -4-thiazoline-2-thione 399 -4-thiazoline-2-thione 399
Acetophenone, in Mannich reaction 44
Acetophenone, in synthesis of amines 14
Acetyl chloride, with aminothiazoles 48
Acetylated amino thiazoles see Acetamidothiazoles
Acetylations see Acylations
Acetylimino-4-thiazolines 91 283—291
Acid (carboxylic), in heterocyclization s 129
Acid (carboxylic), to acylaminothiazoles 90
Acid (carboxylic), with 2-aminothiazoles 47
Acid catalysis 32 38
Acid catalysis in ammonolysis of halothiazoles 13
Acid catalysis in nitrations 73
Acid catalysis of chelate formation 95
Acid catalysis of heterocyclization 91
Acid catalysis of hydrolysis 92 99 105
Acid catalysis of rearrangement 102 130
Acid chlorides, with  -2-thiazoline-5-one anion 432 -2-thiazoline-5-one anion 432
Acid chlorides, with  -4-thiazoline-2-thione anion 396 -4-thiazoline-2-thione anion 396
Acid chlorides, with 3-amino-2-iminothiaz-oline 131
Acid chlorides, with aminothiazoles 48 90
Acid halides, with aminothiazoles 47
Acidity, of 2-aminothiazoles 90
Acrylic fibers 105
Acrylonitrile, reaction of, with ambident anion of  -4-thiazoline-2-thione 394 -4-thiazoline-2-thione 394
Acrylophenone, with aminothiazoles 42
Activation, by amino group of nucleophilic substitution 18
Activation, by nitro group 12
Activation, by protomeric equilibrium 18
Acyl-2-imino-4-thiazolines, reduction of 127
Acylaminothiazoles 90 220—241
Acylaminothiazoles from acids 90
Acylaminothiazoles from acyl chloride 90
Acylaminothiazoles from anhydrides 90
Acylaminothiazoles from azidothiazoles 90
Acylaminothiazoles, 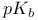 of 91 of 91
Acylaminothiazoles, alkylation, general patterns 91
Acylaminothiazoles, by heterocyclization 90
Acylaminothiazoles, Curtius degradation to 90
Acylaminothiazoles, hydrolysis of 92
Acylaminothiazoles, Mannich bases from 91
Acylaminothiazoles, mass spectra of 91
Acylation of  -4-thiazoline-2-one 402 -4-thiazoline-2-one 402
Acylations 47
Additive, for antifouling point 440
Additive, for lubricant 442
Additive, for oil 438
Adrenergic activities, of 1-alkylamino-3-(2 thiazoloxy)-2-propanol derivatives 410
Adrenergic activities, relation with conformation in 410
Adsorption 170
Agricultural applications 132
Alcohol, by oxydation 45
Alcohol, in alkylations, of  -4-thiazoline-2-thiones 392 -4-thiazoline-2-thiones 392
Alcohol, in alkylations, of alkylaminothiazoles 48
Alcohol, in alkylations, of aminothiazoles 38
Aldehydes, with  -2-thiazohne-5-ones 432 -2-thiazohne-5-ones 432
Aldehydes, with aminothiazoles 98
Alga scenedesmus acutus 134
Algicide 438
Alkali amides see Amide ions
Alkenes, in alkylation of  -4-thiazoline-2-thiones 392 -4-thiazoline-2-thiones 392
Alkenes, in cycloaddition with  -2-thiazoline-5-one 436 -2-thiazoline-5-one 436
Alkyl halides, reaction with,  -4-thiazoline-2-thiones anion 392 396 -4-thiazoline-2-thiones anion 392 396
Alkyl-acetamidothiazoles, with benzaldehyde 46
Alkylaminothiazoles, aminoalkylation of 81
Alkylaminothiazoles, from thiazolyl amidines 99
Alkylaminothiazoles, rearrangement of 80
Alkylaminothiazoles, reduction of 87
Alkylaminothiazoles, structure determination of, by chemical methods 37
Alkylaminothiazoles, structure determination of, by spectroscopic methods 38
Alkylaminothiazoles, to mesoionic xanthine 60
Alkylaminothiazoles, with acetic anhydride 55
Alkylaminothiazoles, with alcohols 47
Alkylaminothiazoles, with alkali amides 43
Alkylaminothiazoles, with malonate esters 55
Alkylation, and chemical structure determination 37
Alkylation, and rearrangements 33
Alkylation, at nitrogen in  -4 thiazoline-2-one, reactivity of ambident anion 401 -4 thiazoline-2-one, reactivity of ambident anion 401
Alkylation, at nitrogen in  -4 thiazoline-2-one, selectivity of 401 -4 thiazoline-2-one, selectivity of 401
Alkylation, at nitrogen in  -4-thiazoline-2-thione, in relation with ambident reactivity 394—396 -4-thiazoline-2-thione, in relation with ambident reactivity 394—396
Alkylation, at nitrogen in  -4-thiazoline-2-thione, reaction with diazoalkanes 394 -4-thiazoline-2-thione, reaction with diazoalkanes 394
Alkylation, at nitrogen in  -4-thiazoline-2-thione, reaction with methylvinylketones 394 -4-thiazoline-2-thione, reaction with methylvinylketones 394
Alkylation, at oxygen in  -2-thiazoline-4-one 423 -2-thiazoline-4-one 423
Alkylation, at oxygen in  -4-thiazoline-2-one, diazomethane reaction 401 -4-thiazoline-2-one, diazomethane reaction 401
Alkylation, at oxygen in  -4-thiazoline-2-one, steric effect on 401 -4-thiazoline-2-one, steric effect on 401
Alkylation, at sulfur in  -2-thiazoline-5-thione 416 -2-thiazoline-5-thione 416
Alkylation, at sulfur in  -4-thiazoline-2-thione, preparative aspects, with alcohol 392 -4-thiazoline-2-thione, preparative aspects, with alcohol 392
Alkylation, at sulfur in  -4-thiazoline-2-thione, preparative aspects, with alkenes 392 -4-thiazoline-2-thione, preparative aspects, with alkenes 392
Alkylation, at sulfur in  -4-thiazoline-2-thione, preparative aspects, with alkylhalides 391 396 -4-thiazoline-2-thione, preparative aspects, with alkylhalides 391 396
Alkylation, at sulfur in  -4-thiazoline-2-thione, preparative aspects, with diazomethane 395 -4-thiazoline-2-thione, preparative aspects, with diazomethane 395
Alkylation, at sulfur in  -4-thiazoline-2-thione, preparative aspects, with dihalogenoalkanes 391 -4-thiazoline-2-thione, preparative aspects, with dihalogenoalkanes 391
Alkylation, at sulfur in  -4-thiazoline-2-thione, preparative aspects, with methyliodide 391 -4-thiazoline-2-thione, preparative aspects, with methyliodide 391
Alkylation, at sulfur in  -4-thiazoline-2-thione, quantitative aspects see Nucleophilic substitution -4-thiazoline-2-thione, quantitative aspects see Nucleophilic substitution
Alkylation, in aprotic solvents 35
Alkylation, of 2-acetamidothiazoles 35 37
Alkylation, of alkylaminothiazole 34
Alkylation, of alkylaminothiazole, with alcohols 47 80 90
Alkylation, of aminothiazoles, with 2-propynyl bromide 32
Alkylation, of aminothiazoles, with 3-chloropropionic acid 33
Alkylation, of aminothiazoles, with alcohols 38
Alkylation, of aminothiazoles, with benzyl chloride 33
Alkylation, of aminothiazoles, with chloracetic acid 33
Alkylation, of aminothiazoles, with chloracetic esters 33
Alkylation, of aminothiazoles, with dimethylaminoethylchloride 35
Alkylation, of aminothiazoles, with ethyl iodide 33
Alkylation, of aminothiazoles, with ethylene oxide 34 38
Alkylation, of aminothiazoles, with phenethyl chloride 35
Alkylation, of azothiazoles 105
Alkylation, of dialkylaminothiazoles 32
Alkylation, of nitraminothiazoles 37
Alkylation, of sulfathiazoles 116
Alkylation, of thiazolylureas 93
Alkylation, with alkali amides 34 35
Alkylation, with phthalimidoethylbromide 35
Alkylthiazoles, quaternary salts of 15
Alkyne in cycloaddition reaction with  -2-thiazoline-5-one 436 -2-thiazoline-5-one 436
Alkyne in cycloaddition reaction with  -2-thiazoline-5-one, 1,4-dichloro-2-butyne, reaction with -2-thiazoline-5-one, 1,4-dichloro-2-butyne, reaction with  -4-thiazoline-2-thione anion 396 -4-thiazoline-2-thione anion 396
Alkyne in cycloaddition reaction with  -2-thiazoline-5-one, in synthesis of -2-thiazoline-5-one, in synthesis of  -4 thiazoline-2-thione 372 -4 thiazoline-2-thione 372
Allergy prevention 148
Aluminum chloride, isomerization of 5,5-diphenyl-2,4 thiazolidine 373
Aluminum complexes 161
Aluminum-mercury amalgam, reduction of 2-thiazolylsulfide 405
Ambident reactivity and HSAB theory 5
Ambident reactivity and kinetic data 63
Ambident reactivity and molecular orbital calculations 61 62
Ambident reactivity and PES 63
Ambident reactivity and single electron transfer 6
Ambident reactivity and steric effects 63
Ambident reactivity in nitration reactions 72
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



 -4-thiazoline-2-thione and
-4-thiazoline-2-thione and 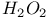
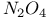
 -diketones
-diketones 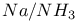 reduction of 4-methyl-
reduction of 4-methyl-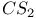
 -chloroacetamide
-chloroacetamide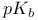 of
of