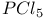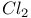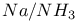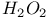|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Metzger J.V. — Thiazole and Its derivatives (Part 2) |
|
|
 |
| Предметный указатель |
 -4-Thiazoline-2-thione, thiocyanation of 400 -4-Thiazoline-2-thione, thiocyanation of 400
 -4-Thiazoline-2-thione, to 2-imino-4-thiazolines 123 -4-Thiazoline-2-thione, to 2-imino-4-thiazolines 123
 -4-Thiazoline-2-thione, transformation in, -4-Thiazoline-2-thione, transformation in,  -4-thiazoline-2-one 374 -4-thiazoline-2-one 374
 -4-Thiazoline-2-thione, transformation in, 2-chlorothiazoles 393 -4-Thiazoline-2-thione, transformation in, 2-chlorothiazoles 393
 -4-Thiazoline-2-thione, transformation in, 2-imino-4-thiazolyl derivatives 398 -4-Thiazoline-2-thione, transformation in, 2-imino-4-thiazolyl derivatives 398
 -4-Thiazoline-2-thione, transformation in, 2-thiazolylsulfonic acid 393 413 -4-Thiazoline-2-thione, transformation in, 2-thiazolylsulfonic acid 393 413
 -4-Thiazoline-2-thione, transformation in, 2-thiazolylsulfonylchloride 393 414 -4-Thiazoline-2-thione, transformation in, 2-thiazolylsulfonylchloride 393 414
 -4-Thiazoline-2-thione, transformation in, 2-unsubstituted thiazoles 413 393 397 -4-Thiazoline-2-thione, transformation in, 2-unsubstituted thiazoles 413 393 397
 -4-Thiazoline-2-thione, transformation in, sulfcnamidothiazoles 411 -4-Thiazoline-2-thione, transformation in, sulfcnamidothiazoles 411
 -4-Thiazoline-2-thione, transformation in, thiazolyl disulfide 393 397 399 -4-Thiazoline-2-thione, transformation in, thiazolyl disulfide 393 397 399
 -4-Thiazoline-2-thione, transition metal complexes 386 -4-Thiazoline-2-thione, transition metal complexes 386
 -4-Thiazoline-2-thione, ultraviolet spectra 378 -4-Thiazoline-2-thione, ultraviolet spectra 378
 -4-Thiazoline-2-thione, uses 438 439 -4-Thiazoline-2-thione, uses 438 439
 -4-Thiazoline-2-thione, UV spectra, CNDO calculation 380 -4-Thiazoline-2-thione, UV spectra, CNDO calculation 380
 -4-Thiazoline-2-thione, UV spectra, effect of self-association on 381 -4-Thiazoline-2-thione, UV spectra, effect of self-association on 381
 -4-Thiazoline-2-thione, UV spectra, PPP calculation 380 -4-Thiazoline-2-thione, UV spectra, PPP calculation 380
 -4-Thiazoline-2-thione, UV spectra, steric effect on 381 -4-Thiazoline-2-thione, UV spectra, steric effect on 381
 -4-Thiazoline-2-thione, X-ray determination 385 386 -4-Thiazoline-2-thione, X-ray determination 385 386
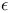 -Caprolactam polymers 438 -Caprolactam polymers 438
 -Chlorobutyryichloride, with aminothiazoles 50 -Chlorobutyryichloride, with aminothiazoles 50
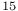 C-NMR C-NMR \Delta$-4-thiazoline-2-one 390 \Delta$-4-thiazoline-2-one 390
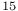 C-NMR C-NMR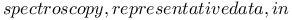 \Delta$-4-thiazoline-2-thione 388 \Delta$-4-thiazoline-2-thione 388
1,2,4-Triazolin, 4-phenyl-3,5-dione 433
1,2,4-Triazolin, from  -2-thiazolin-5-ones 435—436 -2-thiazolin-5-ones 435—436
1,2-Dithiole-3-thione, methylation of 391
1,3,4-Thiadiazole-2-thione, reaction with alkynes 372
1,3,5-Oxadiazine-2,4,6-trione, to biuret derivatives 56
1,3-Diester, of 2-imino-4-thiazoline 123
1,3-Dipolar cyclo additions, of  -2-thiazoline-4-one 425 426 -2-thiazoline-4-one 425 426
1,3-Dipolar cyclo additions, with dimethyl acetylenodicarboxylate 426 427
1,3-Dipolar cyclo additions, with methyl fumarate 425 426
1,3-Dipolar cyclo additions, with methyl maleate 425 426
1,4-Dichloro-2-butene, reaction with,  -4-thiazoline-2-thione anion 396 -4-thiazoline-2-thione anion 396
2,4-Dichlorothiazole, 2,5-dichlorothiazole 408
2,4-Thiazolidinedithione, isomerization with 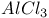 373 373
2,4-Thiazoline diones, from  -2-thiazoline-4-ones 423 424 -2-thiazoline-4-ones 423 424
2-(6'-Hydroxybenzothiazol-2'-yl)-4-hydroxythiazole 420—421
2-Acetylamino, 5-bromo-thiazole, reactionwith sodium thiophenoxide 418
2-Acetylamino-thiazole, reaction with sulfonylchloride derivatives 418
2-Alkylimino-4-thiazolines 127
2-Alkylthio-thiazolium salts, preparation of, from N-substituted  -4-thiazoline-2-thiones and alkylating agents 391 392 -4-thiazoline-2-thiones and alkylating agents 391 392
2-Allyloxythiazole 410
2-Amino-3-thiazolines, by bromination 78
2-Amino-4-methyl-thiazole anion, behavior in Na/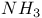 398 398
2-Amino-5-bromothiazole, reaction with hydrogen sulfide 417
2-Aminothiazole, to 2-mercaptothiazoie 370
2-Aminothiazole, with acetaldehyde 42
2-Aminothiazoles 12
2-Aminothiazoles, acidity of 90
2-Aminothiazoles, acylations of, with  -chloropropionylchioride 50 -chloropropionylchioride 50
2-Aminothiazoles, acylations of, with  -chlorobutyrylchloride 50 -chlorobutyrylchloride 50
2-Aminothiazoles, acylations of, with acetic acid 53
2-Aminothiazoles, acylations of, with acetic anhydride 52
2-Aminothiazoles, acylations of, with acyl halides 48
2-Aminothiazoles, acylations of, with chloracetyl chloride 49
2-Aminothiazoles, acylations of, with esters 53
2-Aminothiazoles, acylations of, with ethyl acrylate 54
2-Aminothiazoles, acylations of, with indoiyi derivatives 48
2-Aminothiazoles, acylations of, with malonic esters 55
2-Aminothiazoles, acylations of, with malonyl chloride 49
2-Aminothiazoles, acylations of, with oxalyl chloride 50
2-Aminothiazoles, acylations of, with sodium acetate 52
2-Aminothiazoles, acylations of, with unsaturated acyl chloride 49
2-Aminothiazoles, additions to-double bonds 40
2-Aminothiazoles, alkylations, rate constants of 32
2-Aminothiazoles, alkylations, with 2-chloropropionicacid 32
2-Aminothiazoles, alkylations, with 2-propynyl bromide 32
2-Aminothiazoles, alkylations, with alcohols 38
2-Aminothiazoles, alkylations, with benzyhydryl chloride 34
2-Aminothiazoles, alkylations, with benzyl chloride 80
2-Aminothiazoles, alkylations, with chloracetic acid 33
2-Aminothiazoles, alkylations, with chloracetic esters 33
2-Aminothiazoles, alkylations, with dialkylaminoalkyl halides 33
2-Aminothiazoles, alkylations, with dimethylaminoethylchloride 35
2-Aminothiazoles, alkylations, with ethyl iodide 33
2-Aminothiazoles, alkylations, with ethylene oxide 34 38
2-Aminothiazoles, alkylations, with phenethyl chloride 35
2-Aminothiazoles, alkylations, with tosyl chloride 69
2-Aminothiazoles, ambident reactivity of 30 31 38 39 61—64
2-Aminothiazoles, anaesthetic properties of 42 43
2-Aminothiazoles, and acrylophenone 42
2-Aminothiazoles, and phenone 42
2-Aminothiazoles, basicity of 90
2-Aminothiazoles, biheterocychc structures from 33 42
2-Aminothiazoles, by Chichibabin reaction 12
2-Aminothiazoles, by hydrolysis 92
2-Aminothiazoles, by mercapto group replacement 13
2-Aminothiazoles, colorimetric analysis of 30
2-Aminothiazoles, complexes of 120
2-Aminothiazoles, dialkylation of 37
2-Aminothiazoles, diazotization of 66 67
2-Aminothiazoles, dipole moment of 29
2-Aminothiazoles, electrophilic centers substitution by 32
2-Aminothiazoles, extraction of 30
2-Aminothiazoles, from 2-halothiazoles 12
2-Aminothiazoles, gas-liquid-chromatography of 30
2-Aminothiazoles, in biological samples 30
2-Aminothiazoles, in blood 30
2-Aminothiazoles, in feeds 30
2-Aminothiazoles, in Ullman synthesis 115
2-Aminothiazoles, in urine 30
2-Aminothiazoles, in wine 30
2-Aminothiazoles, magnetic susceptibilities of 29
2-Aminothiazoles, Mannich bases from 91
2-Aminothiazoles, mass spectra of 27
2-Aminothiazoles, metabolite from 42
2-Aminothiazoles, molecular orbital calculations of 61 62
2-Aminothiazoles, NMR spectra of 25 26
2-Aminothiazoles, ozonolysis of 71
2-Aminothiazoles, paper electrophoresis of 30
2-Aminothiazoles, polarographic data 29
2-Aminothiazoles, purification of 30
2-Aminothiazoles, quaternary salts of 32
2-Aminothiazoles, reduction of 91
2-Aminothiazoles, Schiff bases with 30
2-Aminothiazoles, solid state reactions with 52
2-Aminothiazoles, sublimation of 30
2-Aminothiazoles, synthesis of 12
2-Aminothiazoles, thin layer chromatography of 30
2-Aminothiazoles, titration of 30
2-Aminothiazoles, to iminothiazolines 38 47
2-Aminothiazoles, to iminothiazolines, IR spectra of 24
2-Aminothiazoles, to sulfonamido thiazoles 69
2-Aminothiazoles, toward electrophilic reagents 71—84
2-Aminothiazoles, toward electrophilic reagents, alkylation 80
2-Aminothiazoles, toward electrophilic reagents, coupling with diazonium 76
2-Aminothiazoles, toward electrophilic reagents, general trends 87 88
2-Aminothiazoles, toward electrophilic reagents, halogenation of 77
2-Aminothiazoles, toward electrophilic reagents, nitration of 72
2-Aminothiazoles, toward electrophilic reagents, nitrosation of 74
2-Aminothiazoles, toward electrophilic reagents, sulfonation of 75
2-Aminothiazoles, transition metal complexes of 70
2-Aminothiazoles, with  -trichloromethyl- -trichloromethyl- -propiolactone 55 -propiolactone 55
2-Aminothiazoles, with 1-imino-3-amino-isoindolenine 56
2-Aminothiazoles, with 2-chloronicotinic acid 57
2-Aminothiazoles, with 8-hydroxyquinolme 44
2-Aminothiazoles, with aldehydes 98
2-Aminothiazoles, with aromatic aldehydes 40
2-Aminothiazoles, with benzaldehyde 45
2-Aminothiazoles, with caibonyl derivatives 40
2-Aminothiazoles, with carbon disulfide 60
2-Aminothiazoles, with chloracetyl chloride 33
2-Aminothiazoles, with cyanamide 58
2-Aminothiazoles, with dicyandiamide 57
2-Aminothiazoles, with halocarbonyl derivatives 33
2-Aminothiazoles, with isothiocyanate 61 65 93
2-Aminothiazoles, with oxadiazine 56
2-Aminothiazoles, with picryl halides 56
2-Aminothiazoles, with pyridyl formimidate 55
2-Aminothiazoles, with salicylaldehyde 41
| 2-Aminothiazoles, with silicon compounds 68
2-Aminothiazoles, with sulfonamido thiazoles 69
2-Aminothiazoles, with terephthaldehyde 41
2-Aminothiazoles, with thiourea 79
2-Aminothiazoles, with trimethyiene tribromide 33
2-Aminothiazoles, with urea 56
2-Aminothiazoles, X-Ray of 29
2-Anilinothiazoles, with acetic anhydride 53
2-Arylsulfenamidothiazoles, rearrangement of 82
2-Benzylthiothiazoles, transformation in,  -4-thiazoline-2-thiones 405 -4-thiazoline-2-thiones 405
2-Bromo-4-aminothiazole derivatives, biological activity of 138
2-Bromo-4-methylthiazole, reaction with, 2-mercaptothiazole 370
2-Bromo-4-methylthiazole, reaction with, hydrogen sulfide 370
2-Bromothiazole, reaction with thiourea 370
2-Bromothiazole, to  -4-thiazoline-2-thione 370 -4-thiazoline-2-thione 370
2-Chloronicotinic acid, with 2-aminothiazoles 57
2-Chlorothiazole, from  -4-thiazoline-2-one and -4-thiazoline-2-one and 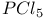 401 401
2-Chlorothiazole, from  -4-thiazoline-2-one and HCl 402 -4-thiazoline-2-one and HCl 402
2-Chlorothiazole, from  -4-thiazoline-2-thione and -4-thiazoline-2-thione and 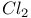 393 393
2-Chlorothiazole, preparation of, from dithiazolylsulfide (HCl) 405
2-Dimethylamino thiazoliufn salts, hydrolysis 373
2-Disubstituted aminothiazoles 259—267
2-Halogenothiazole,  -4-thiazoline-2-thione anion 396 404 -4-thiazoline-2-thione anion 396 404
2-Halogenothiazole, mercaptan 404
2-Halogenothiazole, metal sulfinate 415
2-Halogenothiazole, reaction with, alcohol 408
2-Halogenothiazolium salts, hydrolysis (of) to  -4-thiazoline-one 373 -4-thiazoline-one 373
2-Haloimino-4-thiazolines 127
2-Hydrazinothiazoles 99 248—254
2-Hydrazinothiazoles, bromination of 103 106
2-Hydrazinothiazoles, HMO treatment of 100
2-Hydrazinothiazoles, IR spectra of 106
2-Hydrazinothiazoles, organometallics in preparation of 99
2-Hydrazinothiazoles, oxidation of 102
2-Hydrazinothiazoles, reactions with,  -chloropropionyl chloride 102 -chloropropionyl chloride 102
2-Hydrazinothiazoles, reactions with,  -ketoesters 102 -ketoesters 102
2-Hydrazinothiazoles, reactions with, 1,3-diketones 102
2-Hydrazinothiazoles, reactions with, acetic anhydride 101 106
2-Hydrazinothiazoles, reactions with, arylhalides 101
2-Hydrazinothiazoles, reactions with, benzaldehyde 100
2-Hydrazinothiazoles, reactions with, Grignard reagents 100
2-Hydrazinothiazoles, reactions with, hydrazine 106
2-Hydrazinothiazoles, reactions with, isocyanate 100 106
2-Hydrazinothiazoles, reactions with, mercuric oxide 100
2-Hydrazinothiazoles, reactions with, methyl sulfate 106
2-Hydrazinothiazoles, reactions with, nitrous acid 100
2-Hydrazinothiazoles, reactions with, sugar 101
2-Hydrazinothiazoles, reactions with, thiocyanate 100
2-Hydrazinothiazoles, rearrangement of 101 102 104
2-Hydroxythiazole see  -4-Thiazo]ine-2-one -4-Thiazo]ine-2-one
2-Imino- -4-thiazoline, nitroso derivatives 374 375 -4-thiazoline, nitroso derivatives 374 375
2-Imino- -4-thiazoline, to -4-thiazoline, to  -4-thiazoline-2-one 374 -4-thiazoline-2-one 374
2-Imino-4-thiazolyl derivatives, preparation of, from  -4-thiazoline-2-one 402 -4-thiazoline-2-one 402
2-Imino-4-thiazolyl derivatives, preparation of, from  4-thiazoline-2-thione 398 4-thiazoline-2-thione 398
2-Imino-l,3-oxythiolan derivatives, ring transformation of 374 375
2-Iminothiazole, to  -4-thiazoline-2-thione 37 -4-thiazoline-2-thione 37
2-Iminothiazolidine-4-one, preparation of 424
2-Iminothiazolium salts, to  -4-thiazoline-2-thione 372 -4-thiazoline-2-thione 372
2-Lithium thiazolyl, reaction with, disulfides 404
2-lmino-4-thiazolines 274—283
2-lmino-4-thiazolines, acetylation of 124 132
2-lmino-4-thiazolines, alkylation of 129
2-lmino-4-thiazolines, by nitration 74
2-lmino-4-thiazolines, coupling with diazoniums 128
2-lmino-4-thiazolines, dipole moment of 29
2-lmino-4-thiazolines, from aminothiazoles 38 47
2-lmino-4-thiazolines, from aminothiazolium salts 32
2-lmino-4-thiazolines, hydrolysis of 130
2-lmino-4-thiazolines, nitration of 125
2-lmino-4-thiazolines, nitrosation of 125
2-lmino-4-thiazolines, NMR spectra of 25 38 26
2-lmino-4-thiazolines, rearrangement of 130
2-lmino-4-thiazolines, sulfonation of 124
2-lmino-4-thiazolines, synthesis of 122
2-lmino-4-thiazolines, to 1,3,4-oxadiazoles, protomeric studies by IR 24
2-lmino-4-thiazolines, to disulfonamido thiazoles 69
2-lmino-4-thiazolines, to thioureas 125
2-lmino-4-thiazolines, UV spectra of 38
2-lmino-4-thiazolines, versus 2-aminothiazoles identification 35 38
2-lmino-4-thiazolines, with  -trichloromethyl- -trichloromethyl- -propiolactone 55 -propiolactone 55
2-lmino-4-thiazolines, with 2-chlorothiazole 57
2-lmino-4-thiazolines, with carbon disulfide 126
2-lmino-4-thiazolines, with isocyanates 125
2-lmino-4-thiazolines, with isothiocyanates 58 125
2-Meicaptothiazoles in synthesis of 2-aminothiazoles 13
2-Meicaptothiazoles, reaction with hydrazine 99
2-Meicaptothiazoles, reaction with morpholine 13
2-Mercaptothiazole see  -4-Thiazoline-2-thione -4-Thiazoline-2-thione
2-Methylamino-4-methylthiazole anion, behavior in 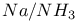 398 398
2-Methylthio-3-methylthiazolium salts, as catalyst for methylthiothiazole rearrangement 406
2-Nitroresorcinol, coupling with 112
2-Propynyl bromide, quaternarization with aminothiazole 32
2-Pyridone, derivatives, from  -2-thiazoline-4-one 426—427 -2-thiazoline-4-one 426—427
2-Substituted aminothiazole 211—212
2-Sulfenamidothiazoles (or 2-sulfenylthia-zoles), rearrangement of 114
2-Sulfenamidothiazoles (or 2-sulfenylthia-zoles), synthesis of 69 113
2-Sulfenyl thiazoles see Sulfenamidothiazoles
2-Thiazolyl diazonium 109
2-Thiazolyl diazonium by diazotization of aminothiazoles 110
2-Thiazolyl diazonium with potassium ethylxanthate 370
2-Thiazolyl diazonium with potassium thiocyanatc 370 408
2-Thiazolyl diazonium with thiourea 370
2-Thiazolyl diazonium with water 375
2-Thiazolyl diazonium, diazo coupling with 110 112
2-Thiazolyl diazonium, ionic scission of 110
2-Thiazolyl diazonium, radicals from 110
2-Thiazolyl diazonium, reactions 110 111 112
2-Thiazolyl disulfides, physical properties 412
2-Thiazolyl disulfides, table of compounds 468—471
2-Thiazolyl disulfides, uses of 441
2-Thiazolyl dithio carbamates 96
2-Thiazolyl dithio carbamates, synthesis of 60 246
2-Thiazolyl dithiocarbonic acids and esters 246
2-Thiazolyl oxides, nitration 410
2-Thiazolyl oxides, physical properties, mass spectra 409
2-Thiazolyl oxides, physical properties, NMR 409
2-Thiazolyl oxides, physical properties, pKa 409
2-Thiazolyl oxides, physical properties, UV 409
2-Thiazolyl oxides, preparation of, alkylation of  -4-thiazoline-2-ones 408 -4-thiazoline-2-ones 408
2-Thiazolyl oxides, preparation of, Williamson reactions 408
2-Thiazolyl oxides, reaction with methoxide ion (nucleophilic addition) 410
2-Thiazolyl oxides, rearrangement of, 2-allyloxythiazole 410
2-Thiazolyl oxides, rearrangement of, 2-methoxythiazole 409
2-Thiazolyl oxides, tables of compounds, 4,5-disubstituted derivatives 511—512
2-Thiazolyl oxides, tables of compounds, 4-substituted derivatives 507—508
2-Thiazolyl oxides, tables of compounds, 5-substituted derivatives 509—510
2-Thiazolyl oxides, tables of compounds, unsubstituted derivatives 505—506
2-Thiazolyl oxides, uses of 441
2-Thiazolyl sulfenyl chloride, transformation to, thiazolyl disulfides 412
2-Thiazolyl sulfide, in hydrocarbon synthesis 406
2-Thiazolyl sulfide, oxidation of, with  415 415
2-Thiazolyl sulfide, oxidation of, with 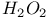 405 415 405 415
2-Thiazolyl sulfide, oxidation of, with 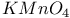 415 415
2-Thiazolyl sulfide, oxidation of, with m-chloroperbenzoic acid 415
2-Thiazolyl sulfide, physical properties, infrared 405
2-Thiazolyl sulfide, physical properties, NMR 404
2-Thiazolyl sulfide, physical properties, pKa 404
2-Thiazolyl sulfide, physical properties, ultraviolet 404
2-Thiazolyl sulfide, preparation of, from  -4-thiazoline-2-thione and alcohols 391 -4-thiazoline-2-thione and alcohols 391
2-Thiazolyl sulfide, preparation of, from  -4-thiazoline-2-thione and alkenes 392 -4-thiazoline-2-thione and alkenes 392
2-Thiazolyl sulfide, preparation of, from  -4-thiazoline-2-thiones and halo-derivatives 391 392 404 -4-thiazoline-2-thiones and halo-derivatives 391 392 404
2-Thiazolyl sulfide, preparation of, from 2-halothiazoles and mercaptans 403—404
2-Thiazolyl sulfide, preparation of, from 2-lithium thiazolyl and disulfides 404
2-Thiazolyl sulfide, reaction with, haloalkanes (kinetics of) 405
2-Thiazolyl sulfide, rearrangement in N-alkyl- -4-thiazoline-2-thiones 406 -4-thiazoline-2-thiones 406
2-Thiazolyl sulfide, reduction of, by aluminum mercury amalgam 405
2-Thiazolyl sulfide, reduction of, by zinc powder 405
2-Thiazolyl sulfide, ring opening in 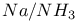 406 406
2-Thiazolyl sulfide, tables of compounds, 4,5-disubstituted 456—467
2-Thiazolyl sulfide, tables of compounds, 4-substituted 445—449
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



 -4-Thiazoline-2-thione, thiocyanation of
-4-Thiazoline-2-thione, thiocyanation of  -Caprolactam polymers
-Caprolactam polymers  -Chlorobutyryichloride, with aminothiazoles
-Chlorobutyryichloride, with aminothiazoles  C-NMR
C-NMR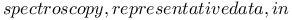 \Delta$-4-thiazoline-2-one
\Delta$-4-thiazoline-2-one 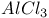
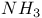
 -chloropropionylchioride
-chloropropionylchioride