|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Metzger J.V. — Thiazole and Its derivatives (Part 2) |
|
|
 |
| Предметный указатель |
Oxalate 121
Oxalyl chloride, with aminothiazoles 50
Oxidation of  -4-thiazoline-2-thione 374 393 413 -4-thiazoline-2-thione 374 393 413
Oxidation of 2-thiazolyl sulfides 405 414 415
Oxidation of 5-thiazolyl sulfides 415 418
Oxidation of 5-thiocyanato-thiazole 418
Oxidation of leucobase 45
Oxidation of nitrosulfonamides 115
Oxidation of Photinus pyralis luciferin 420
Oxidation, of hydrazinothiazoles 102
Oxime of  -2-thiazoline-4-one 425 -2-thiazoline-4-one 425
Oxime, Beckmann rearrangement of 16
p-Dimethylaminobenzaldehyde, reaction with 2-methyl- -2-thiazoline-4-one 425 -2-thiazoline-4-one 425
P-Dimethylaminoethyl chloride, reaction with,  -4-thiazoline-2-thione anion 396 -4-thiazoline-2-thione anion 396
p-Nitrobenzenesulfenylchloride 403 418
P.E.S. 21
P.E.S. and CNDO calculations 22
P.E.S. and kinetic data 63
P.E.S.,  -4-thiazoline-2-thione 381 -4-thiazoline-2-thione 381
P.P.P. approximation, and U.V. spectra 21
P.P.P. approximation, charge diagrams calculations by 17
P.P.P. approximation, of  -4-thiazoline-2-one 389 390 -4-thiazoline-2-one 389 390
P.P.P. approximation, of  -4-thiazoline-2-thione 389 -4-thiazoline-2-thione 389
Paints 170
Palladium complexes 108 155 159 161
Paper-chromatography, of 2-aminothiazoles 30 124
Paper-chromatography, of 2-iminothiazolines 124
Paper-chromatography, of complexes 104
Paper-chromatography, of sulfathiazole 116
Paper-chromatography, of thiazolyl thioureas 94
Paper-electrophoretic 107
Paraformaldehyde 129
Paraformaldehyde in synthesis of amines 14
Parasiticide 133
Parkinsonism 152
Penicillin derivatives 152
Perbromides, in bromination 77
Perchlorate 121
Peroxide, in mechanism of luciferin oxidation 421
Pesticide 134 137 440 441 442
pH, in ambident reactivity, influence in paper electrophoresis 30
Pharmaceutical applications 132
Phase transfer catalysis 432
Phenethylchloride, with 2-aminothiazole 35
Phenones, with aminothiazoles 42
Phenyl lithium 429
Phenylhydrazone, of  -2-thiazoline-4-one 425 -2-thiazoline-4-one 425
Phenylmagnesium bromide 428
Phenylmagnesium bromide with 2-azidothiazoles 113
Phosphate, chlorodiethylthio 401
Phosphate, chlorodietliyl 401
Phosphoric acid, diazotization with 66
Phosphorous compounds, with 2-aminothiazoles 68 69
Phosphorus oxychloride, reaction with,  -2-thiazoline-4-one 423 -2-thiazoline-4-one 423
Phosphorus pentachloride,  -4-thiazoline-2-one 402 -4-thiazoline-2-one 402
Phosphorus pentachloride, reaction with 401
Phosphorus pentasulfide, in transformation of  -4-thiazoline-one to thiazoline-thione 373 -4-thiazoline-one to thiazoline-thione 373
Phosphorus tribromide 426
Photochemical rearrangement, of 4-hydroxy-thiazolium innersalt 375
Photochemistry, mass spectra fragmentation analogy with, of 2-imino-4-thiazolines 27
Photoelectron spectroscopy see P.E.S.
Photographic applications 132 159
Photographic emulsions 439
Photographic processes 438 440 441
Photographic processes with metal salts of  -4-thiazoline-2-thiones 412 -4-thiazoline-2-thiones 412
Photographic processes with seleno-2-thiazole 370
Photographic processes with thiazolyl disulfides 412
Photometric studies 108
Photosensitive 159
Phthalic anhydride, as protecting group 104
Phthalimidoethyl bromide, alkylation with 35
Physical methods 29
Physiochemical properties, of aminothiazoles 17
Phytopathogenic microorganisms 136
Picrates, of amidines 99
Pigments 156—168
Piperidine 46
Piscaine 146
Plant growth regulator 133 134 137
Plastics 170
Platelet aggregation inhibition 145 150 152
Platinum complexes 161
Polarographic studies 153 154
Polarographic studies of 2-aminothiazoles 29
Polarographic studies of 2-anilinothiazoles 30
Polarographic studies of 2-azothiazoles 107 108
Polyacrylonitrile 105 163 164 167
Polyamines 156—168
Polycaprolactam 156—168
Polyesters 163 165 166 167
Polyhalogenothiazole, reactivity of 408—409
Polymers, 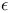 -caprolactam, butadiene 438 -caprolactam, butadiene 438
Polymorphism, of sulfathiazole 116
Positive colored image 440
Postemergence activity 134
Potassium salts, of acetamido thiazoles 90
Potentiometric measurements and amino-imino equilibrium 21
Potentiometric measurements, and amido-imido equilibrium 116
Preemergence herbicide 137
Propargyl amine, reaction with carbon disulfide 372
Protactinium complexes 161
Protecting group, phthalic anhydride as 104
Protective action 170
Proteus vulgaris 152
Protomeric equilibrium 18
Protomeric equilibrium and HMO treatment, 2 2-hydroxythiazole 377
Protomeric equilibrium with thiazolyl carbamates 97 see
Protomeric equilibrium,  -2-thiazoline-4-ones, infrared 421 -2-thiazoline-4-ones, infrared 421
Protomeric equilibrium,  -2-thiazoline-4-ones, NMR study 421 422 -2-thiazoline-4-ones, NMR study 421 422
Protomeric equilibrium,  -2-thiazoline-4-ones, solvent effect 412 -2-thiazoline-4-ones, solvent effect 412
Protomeric equilibrium,  -2-thiazoline-5-ones, infrared 430 -2-thiazoline-5-ones, infrared 430
Protomeric equilibrium,  -2-thiazoline-5-ones, infrared, NMR 430 -2-thiazoline-5-ones, infrared, NMR 430
Protomeric equilibrium,  -2-thiazoline-5-ones, infrared, solvent effect 430—431 -2-thiazoline-5-ones, infrared, solvent effect 430—431
Protomeric equilibrium,  -2-thiazoline-5-ones, infrared, substituent effect 430—431 -2-thiazoline-5-ones, infrared, substituent effect 430—431
Protomeric equilibrium,  -2-thiazoline-5-ones, infrared, U.V. 431 -2-thiazoline-5-ones, infrared, U.V. 431
Protomeric equilibrium,  -2-thiazoline-5-thione 417 -2-thiazoline-5-thione 417
Protomeric equilibrium,  -4-thiazoline-2-one, dipole moment study of 389 -4-thiazoline-2-one, dipole moment study of 389
Protomeric equilibrium,  -4-thiazoline-2-one, infrared spectra 387 -4-thiazoline-2-one, infrared spectra 387
Protomeric equilibrium,  -4-thiazoline-2-one, NMR study 389 -4-thiazoline-2-one, NMR study 389
Protomeric equilibrium,  -4-thiazoline-2-one, solvent effect on 389 -4-thiazoline-2-one, solvent effect on 389
Protomeric equilibrium,  -4-thiazoline-2-one, ultra-violet spectra 387 -4-thiazoline-2-one, ultra-violet spectra 387
Protomeric equilibrium, 2-mercaptothiazole 377
Protomeric equilibrium, acetamido-acetimido 91
Protomeric equilibrium, associated species in 5
Protomeric equilibrium, azohydrazone-hydrazone 107
Protomeric equilibrium, biprotomeric 1
Protomeric equilibrium, determination of pK, from  in in  -4-thiazoline-2-one 389 -4-thiazoline-2-one 389
Protomeric equilibrium, general 1
Protomeric equilibrium, in  -4-thiazoline-2-thione, HMO treatment of substituent effect 377 378 -4-thiazoline-2-thione, HMO treatment of substituent effect 377 378
Protomeric equilibrium, in  -4-thiazoline-2-thione, IR studies 378 -4-thiazoline-2-thione, IR studies 378
Protomeric equilibrium, in  -4-thiazoline-2-thione, NMR studies 379 -4-thiazoline-2-thione, NMR studies 379
Protomeric equilibrium, in  -4-thiazoline-2-thione, solvent effect 379 -4-thiazoline-2-thione, solvent effect 379
Protomeric equilibrium, in  -4-thiazoline-2-thione, steric effect on 380 -4-thiazoline-2-thione, steric effect on 380
Protomeric equilibrium, in  -4-thiazoline-2-thione, ultra violet spectra 378 -4-thiazoline-2-thione, ultra violet spectra 378
Protomeric equilibrium, medium effects on 5
Protomeric equilibrium, substituent effects on 2
Protozoicide 140 440
Pseudomonas aeruginosa 152
Pseudooctahedral stereochemistry 120
Psychotropics 146 147
Purification, of 2-aminothiazoles 30
Pyridine 421
Pyridines 55 57 58 71 79 81
Pyridines, coupling with 112
Pyridines, nitration of 72
Pyridones, from diazonium salts 67
Pyrimidin derivatives 54 56 57 58 119 123 124
Pyrocatechol 155
Pyrocatechol, coupling with 112
Quaternarization see Alkylation
| Quaternary salts, in synthesis of aminothiazoles 15
Quaternary salts, in synthesis of imino-4-thiazolines 32 122
Radical intermediates from azothiazoles 108
Radical intermediates in rearrangement 83
Radical intermediates with lead dioxide 101
Radical intermediates, amino free radical 31 71
Radical intermediates, radical anions 87
Radioactive radiations, protection against 439
Radioprotective action 148 152
Raman spectra of  -4-thiazoline-2-thione 381 -4-thiazoline-2-thione 381
Raman spectra of 2-aminothiazoles 24
Raney nickel 40 86
Raney nickel in relation with 4-thiazole sulfonic acid 413
Raney nickel, desulfurization of  -4-thiazoline-2-thione 399 -4-thiazoline-2-thione 399
Raney nickel, desulfurization of 5-acetylthiothiazoles 417
Rat paw edema inhibition 144
Rate constants and steric effects 32
Rate constants of 2-dialkylaminothiazoles with %lCH_3$ 32
Rate constants of quaternarization of 2-aminothiazoles 32
Rate constants, of coupling 112
Rearrangement 70
Rearrangement in alkylation reactions 34
Rearrangement in diazonium coupling 77
Rearrangement in nitrosatiori 68
Rearrangement of 2-imino-4-thiazolines 130
Rearrangement of 3-aminomethyl to 5-aminomethyl- -4-thiazohne-2-thione 400 -4-thiazohne-2-thione 400
Rearrangement of 5-aminothiazoles 86
Rearrangement of alkylaminothiazoles 80
Rearrangement of hydrazinothiazoles 102 104
Rearrangement of nitramino intermediates 73 113
Rearrangement of sulfenamidothiazoles 82
Rearrangement of sulfonamide thiazoles 69
Rearrangement of thiazolylthioureas 95
Rearrangement sulfonic-sulfamic acid 70 75
Reduction by 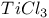 16 16
Reduction by activated iron 16
Reduction by Na in 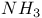 86 86
Reduction by Zn 16 74
Reduction of  -4-thiazoline-2-one with Zn dust 402 -4-thiazoline-2-one with Zn dust 402
Reduction of 2-thiazolylthioether 405
Reduction of 4-alkylidene- 2-thiazoline-5-one 427 428 2-thiazoline-5-one 427 428
Reduction of 5-acetylthio-thiazole 417
Reduction of 5-thiocyanato thiazole 416
Reduction of alkylaminothiazoles 87 88
Reduction of dimethylsulfoxide 376
Reduction of heterocyclic systems 86 115
Reduction of thiazolylamidines 99
Reduction with sodium borohydride 91
Reduction with sodium lithium hydride 92
Reduction, of 2-acetamidothiazole sulfonyl chloride 413
Reimer — Tiemann reactions, on  -4-thiazo-line-2-ones 402 -4-thiazo-line-2-ones 402
Retro — Hantzsch, mechanism of 84 85 102
Rhodanine, alkylation of 419
Rhodanine, ambident reactivity of 419
Rhodanine, reaction with aryldiazonium salts 419
Rhodanine, reaction with halogenothiazoles 79
Rice cultures 135 136 137
Ring opening, of  -2-thiazoline-5-one 433 -2-thiazoline-5-one 433
Ring transformation,  -2-thiazohne-5-one 417 418 -2-thiazohne-5-one 417 418
Ring transformation,  -4-thiazoline-2-thione 373 -4-thiazoline-2-thione 373
Ring transformation, 1,3,4-thiadiazole-2-thione to  -4-thiazo-line-2-thione 372 -4-thiazo-line-2-thione 372
Ring transformation, 1,3-dithiole-2-thione to  -4-ihiazoline-2-thione 372 -4-ihiazoline-2-thione 372
Ring transformation, 2-imino-1,3-oxythiolan derivatives to  -4-thiazoline-2-one 374 375 -4-thiazoline-2-one 374 375
Ring transformation, 5 amino- -4-thiazo-line-2-thione to 4-mercapto-imidazoline-2-thione 399 -4-thiazo-line-2-thione to 4-mercapto-imidazoline-2-thione 399
Ring transformation, 5,5-diphenyl-2,4-thiazolidinedithione, to 4,5-diphenyl- -4-thiazoline-2-thione 373 -4-thiazoline-2-thione 373
Ring transformation, 5-methylenethiazolidine-2-thione to  -2-oxazoline-5-one 417 418 -2-oxazoline-5-one 417 418
Ring-chain tautomerism 113
Rubber, synthetic 439 441
Ruthenium complexes 161
Salicylaldehyde, Schiff base with 99
Salmonella typhi 152
Salts, of acetamido thiazoles 91
Sandmeyer reaction, in halothiazoles synthesis 12
Schiff bases 256—259 268 270
Schiff bases from amiriothiazoles 98
Schiff bases in reduction by 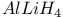 14 14
Schiff bases in reduction by 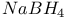 14 14
Schiff bases to amines 40
Schiff bases with 2-amino thiazoles 30 40
Schiff bases, complexes of 99
Schiff bases, cyclization to 42
Schiff bases, IR spectra of 41
Schiff bases, UV spectra of 41 see
Schistosomacidal 141
Schistosomiasis japonica 145
Schotten — Bauman reaction 51 123
Sedative 145 148 438
Selective herbicide 135
Self association, effect of,  -4-thiazoline-2-one, relation with protomery 377 -4-thiazoline-2-one, relation with protomery 377
Self association, effect of,  -4-thiazoline-2-thione, relation with protomery 377 -4-thiazoline-2-thione, relation with protomery 377
Self association, effect of,  -4-thiazoline-2-thione, substituent effect on 384 -4-thiazoline-2-thione, substituent effect on 384
Self association, effect of, on UV spectra of  -4-thiazoline-2-thione 381 -4-thiazoline-2-thione 381
Sigma values, correlation with  19 19
Sigma values, correlation with  , and IR spectroscopy 24 , and IR spectroscopy 24
Silicon compounds, with 2-aminothiazoles 68
Silk 163
Silver complexes 104 108
Silver halide emulsions, miscellaneous 438
Silver halide emulsions, with seleno-2-thiazole 370
Silver nitrate 108
Sodium acetate, catalysis by 81
Sodium acetate, rearrangements with 130
Sodium acetate, to 2-acetamidothiazoles 52
Sodium acetate, with thiazolylureas 93
Sodium amide, in 2-aminothiazole alkylation 35
Sodium amide, in Chichibabin reaction 12
Sodium azide, on diazonium salts 113
Sodium bicarbonate, in preparation of sulfathiazoles 115
Sodium bicarbonate, in rearrangement 86 130
Sodium borohydride 428 429
Sodium borohydride, reduction of 2-aminothiazoles 91
Sodium borohydride, reduction of Schiff bases 14
Sodium carbonate, in preparation of sulfathiazoles 115
Sodium cyanide, in synthesis of 5-amino-thiazole 16
Sodium ethoxide 35
Sodium hydride 37 50 93 432
Sodium lithium hydride, reduction with 92
Sodium nitrite, with 2-imino-4-thiazolines 126
Sodium nitrite, with azothiazoles 111
Sodium salts, of acetamido thiazoles 90
Sodium t-butylate 61
Sodium, in 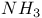 86 86
Sodium-mercury amalgam, reaction with, 2-thiazolyl sulfonic acid 414
Soft acid or base see HSAB theory
Solid state reactions 52
Solvent effect and UV spectra 21
Solvent effect in diazotization 68
Solvent effect in extraction 154
Solvent effect in halogenation 77
Solvent effect in paper chromatography 30
Solvent effect in tautomerism 113
Solvent effect on protomerism, of  -2-thiazoline-4-ones 422 -2-thiazoline-4-ones 422
Solvent effect on protomerism, of  -2-thiazoline-5-ones 430 -2-thiazoline-5-ones 430
Solvent effect on protomerism, of  -4-thiazoline-2-one 389 -4-thiazoline-2-one 389
Solvent effect on protomerism, of  -4-thiazoline-2-thione 379 -4-thiazoline-2-thione 379
Solvent effect on rearrangement 82
Solvent effect, aprotic solvents in alkylation 35
Solvent effect, on alkylation of  -4-thiazo-line-2-thione, with diazomethane 395 -4-thiazo-line-2-thione, with diazomethane 395
Solvent effect, on ambident reactivity 40
Solvent effect, on ambident reactivity, of rhodanine 419
Spectroscopic properties 17 154 155 156 157 158 160 161
Spectroscopic properties of alkylated aminothiazoles 38
Spectroscopic properties of alkylated iminothiazolines 38
Spectroscopic properties of aminothiazoles 17
Spin 121 122
Spot test reagents 150 155 156—168
Stabilizer 170
Stain, complexes of 120
Stain, hydrogenation with 116
Staphylococcus aureus 139 140 141 152
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



 -4-thiazoline-2-thione
-4-thiazoline-2-thione  -caprolactam, butadiene
-caprolactam, butadiene  in
in