|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Metzger J.V. — Thiazole and Its derivatives (Part 2) |
|
|
 |
| Предметный указатель |
Ambident reactivity of  -4-thiazoline-2-one anion, examples 401 -4-thiazoline-2-one anion, examples 401
Ambident reactivity of  -4-thiazoline-2-thione anion 394 -4-thiazoline-2-thione anion 394
Ambident reactivity of  -4-thiazoline-2-thione anion, in relation with HSAB classification 397 -4-thiazoline-2-thione anion, in relation with HSAB classification 397
Ambident reactivity of  -4-thiazoline-2-thione anion, in relation with Klopman — Hudson approach 397 -4-thiazoline-2-thione anion, in relation with Klopman — Hudson approach 397
Ambident reactivity of  -4-thiazoline-2-thione anion, steric effect on 394 -4-thiazoline-2-thione anion, steric effect on 394
Ambident reactivity of hydrazinothiazoles 100
Ambident reactivity of nucleophilic species 68
Ambident reactivity of rhodanine 419
Ambident reactivity of sulfathiazoles 116 117
Ambident reactivity, and Edwards equation 5
Ambident reactivity, general features 61
Ambident reactivity, in alkylations, by alcohols 38 39
Ambident reactivity, in alkylations, of acetamidothiazoles 37
Ambident reactivity, in alkylations, of anions 36 37
Ambident reactivity, in alkylations, of nitraminothiazoles 37
Ambident reactivity, in alkylations, of sulfathiazole 37
Ambident reactivity, of 2-aminothiazoles 30
Ambident reactivity, of electrophilic centers 31 39 126
Ambident reactivity, of electrophilic centers, in new heterocyclic synthesis 33
Ambident reactivity, of electrophilic centers, toward ambident nucleophiles 61
Ambilhar 138 145
Amebicidal 141
Amide ions, alkylaminothiazoles 34
Amide ions, from 2-aminothiazoles 35
Amides, amide-imide tautomerism 116
Amides, with 2-aminothiazoles 47
Amines 403
Amines in chemical structure determination 37
Amines, nitrosation of secondary 40
Amines, preparation of secondary and tertiary 13
Amines, reaction on, 2-alkylthio- -2 thiazoline-4- one 424 -2 thiazoline-4- one 424
Amines, reaction on, 2-benzylthio-oxo- -2-thiazoline-5-one 433 -2-thiazoline-5-one 433
Amines, reaction on, 2-chloro- 2-thiazoline-4-one 424 2-thiazoline-4-one 424
Amines, reaction on, 2-thiazolylsulfonic acid 414
Aminium salts, acylation reagent 132
Aminium salts, alkoxycarbonylation reagent 132
Amino acids, in synthesis of  -2-thiazoline-5-ones 426 -2-thiazoline-5-ones 426
Amino acids, reaction on 4-substituted-2-phenyl- -2-thiazoline-5-ones 433 -2-thiazoline-5-ones 433
Amino-imino equilibrium, and  19 19
Amino-imino equilibrium, and activation of reactivity 18
Amino-imino equilibrium, and HMO treatment 17 19 20 100
Amino-imino equilibrium, and IR 23
Amino-imino equilibrium, and NMR 25
Amino-imino equilibrium, and potentiometry 21
Amino-imino equilibrium, and UV 19 20.
Amino-imino equilibrium, for excited states 21
Amino-imino equilibrium, molecular orbital calculations of 17
Aminoalkylation, of 2-aminothiazoles 81
Aminoalkylation, of acetaminotfuazoles 44
Aminopyridine, and ambident reactivity 63
Aminopyridine, and HSAB 63
Aminopyridine, bromination of 79
Aminopyridine, by Chichibabin reaction 12
Aminopyridine, diazotization of 67
Aminopyridine, nitration of 72
Aminothiazoie-N-oxide 118
Ammonia 115
Ammonia towards radical anion 87
Ammonia with sodium 86
Ammonium chloride, catalysis by 56
Ammonolysis of 2-halothiazoles 12
Amyl nitrite, deamination by 112
Analgesic activity 144 145 147 438 440 442
Analytical uses 30 153 156—168
Analytical uses of azothiazoles 107 154
Analytical uses of chelates 95
Anesthetic activity 42 43 145 149
Angina, treatment of 439
Anhydrides, to acylaminothiazoles 90
Anhydrides, with 2-aminothiazoles 47
Anhydrides, with sulfa thiazoles 117
Aniline, by hydrolysis 130
Aniline, rearrangements in 70 83
Aniline, to 4-aminothiazoles 16
Anions, ambident reactivity of 36
Anions, from thiazolylureas 93
Anions, in 2-aminothiazoles alkylation 34
Anions, of 2-imino-4-thiazoline 127
Anorectic 147
Anthelmintic activity 126 137 430 440 441
Antiamebic activity, of 5-nitro-2-aminothiazoles 72
Antiarrhythmics 441
Antibacterial see Bactericide
Anticholesteremic 150
Anticonvulsant 438
Antifoggant 438
Antifungal agent 139. See also Fungicides
Antihistaminic agent 149
Antihistomonal activity, of 5-nitro-2-aminothiazoles 72
Antiinfective agent 148
Antiinflammatory 138 144 145 438 440 442
Antilipemic 150
Antimalarial agent 140
Antimicrobial 138 141
Antimimotic 152
Antimonytrichloride, with azothiazoles 111
Antimutagenic activity 152
Antineoplastics 76
Antioxidant 170 438
Antiparasitic 136 137.
Antiphlogistic 440 442
Antiprotozoan agent 139
Antipyretic activity 144 145 147 148 440 442
Antischistosomal activity, of 5-nitro-2-aminothiazoles 72
Antispasmodic activity 150 439
Antitrichomonal activity 138
Antitrichomonal activity of 5-nitio-2-aminothiazoles 72
Antitubercular activity 139 140 441 442
Antitumor activity 149 152
Antitussive 144 148
Antiulcer properties 148
Antiviral 138 139 140 144 438
Appetite depressant 147
Aprotic solvent see Solvent effect
Aromatic aldehydes, with aminothiazoles 40
Aryl amines 403
Aryl diazonium salts,  -2-thiazoline-4-ones 425 -2-thiazoline-4-ones 425
Aryl diazonium salts, reaction with, rho-danine 419
Aryl halides,  -4-thiazoline-2-thione anion 396 -4-thiazoline-2-thione anion 396
Aryl halides, with 2-hydrazinothiazples 101
Aryl nitroso, reaction with,  -2-thiazoline-4-one 425 -2-thiazoline-4-one 425
Arylsulfenyl chlorides, with 2-aminothiazoles 69 113
Arylsulfonyl halides 98 115
Arylsulfonylbenzimidoyl chlorides, with aminothiazoles 50
Azide, Curtius rearrangement of 15 16.See
Azide, to carbamate 16
Azide, with azothiazoles 111
Azido thiazoles 113
Azido thiazoles, azidothiazole-thiazolotetrazole 113
Azido thiazoles, reactions of 113
Azirines, reaction with isothiocyanates 15
Azo dye 156—168. See also Dyes
Azothiazoles 105 153 170 271—274
Azothiazoles,  of 107 of 107
Azothiazoles, analytical applications 107
Azothiazoles, by diazo coupling 105
Azothiazoles, by oxidation 102 105
Azothiazoles, dyes 107
Azothiazoles, IR spectra of 107
Azothiazoles, NMR data of 105
Azothiazoles, paper electrophoretic 107
Azothiazoles, polarography of 108
Azothiazoles, protomeric equilibrium with 107
Azothiazoles, X-Ray data 107
Bacteria leaf blight 136
Bactericide 134 136 137 138 139 140 438 439 441 442
Bacteriostatic properties 139 152
Bacterium citri, control of 441
Barium thiocyanate 118
Barrier to rotation, around  - -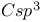 bond, in 3,4-diisopropyl- bond, in 3,4-diisopropyl- 4-thiazoline-2-one 390 4-thiazoline-2-one 390
| Barrier to rotation, around  - -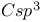 bond, in N-2-propanoic, bond, in N-2-propanoic,  4-thiazoline-2-thione 387 4-thiazoline-2-thione 387
Barrier to rotation, around  - -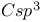 bond, in N-alkyl, bond, in N-alkyl,  -4-thiazoline-2-thione 387 -4-thiazoline-2-thione 387
Base catalysis 34
Base catalysis in acylations 47 48
Base catalysis in hydrolysis 115
Base catalysis of chelate formation 95
Base catalysis of clevage 128
Base catalysis of cy clization 127
Basicity, of 2-aminothiazole 90
Basicity, of 2-imino-4-thiazolines 124
Beckmann rearrangement, of oxime 16
Benzaldehyde, with  -2-thiazoline-4-ones 425 -2-thiazoline-4-ones 425
Benzaldehyde, with alkyl-acetamidothiazole 46
Benzaldehyde, with aminothiazoles 47
Benzaldehyde, with diaminothiazoles 47
Benzaldehyde, with hydrazinothiazoles 100
Benzamide, with 2-bromothiazole 48
Benzene derivatives, coupling with 112
Benzene derivatives, nitrarhino 113
Benzene derivatives, polarographic behavior of 107
Benzhydryl chloride 431
Benzhydryl chloride with 2-aminothiazole 34
Benzidine-like rearrangement 104
Benzimidazole derivatives 57
Benzoin, reaction with,  , 373 , 373
Benzoselenazolium salts, reaction of with  -2-thiazoline-4-one 425 -2-thiazoline-4-one 425
Benzothiazoline-2-thione (N-Me), methylation of 391
Benzothiazolium salts, reaction of, with  -2-thiazoline-4-one 425 -2-thiazoline-4-one 425
Benzoyl chloride 431
Benzoyl chloride with 2-aminothiazoles 48
Benzyl bromide, with thiazolylthiourea 95
Benzyl chloride, with aminothiazoles 33 80
Benzylidene bis aminothiazoles 41
Bidentate ligands 120
Biheterocycles 99. See also Condensed heterocycles
Biological activity 138
Biological activity, 2-aminothiazoles in samples 30
Biological processes 85
Bioluminescence, of firefly lanterns 420
Birch reduction 398
Bisheterocycles 45 46 55 102 104 105 372 373 402 404 408 427 437.
Bismuth complexes 156—168
Biuret, from 2-aminothiazoles 56
Black head in turkeys 148
Blood, 2-aminothiazole in 30
Blood, pharmaceutical use 148
Bromination, of  -4-thiazoline-2 one 402 -4-thiazoline-2 one 402
Bromination, of 2-acetamidothiazoles 79
Bromination, of 2-acetyliminothiazolines 128
Bromination, of 2-aminothiazoles 78 79
Bromination, of 2-azidothiazoles 113
Bromination, of 2-hydrazinothiazoles 103 106
Bromination, of 2-thiazolyl-sulfone 415
Bromination, of 5-thiazolyl-oxide 436
Bromination, with bromine 77
Bromination, with N-bromosuccinimide 77
Bromothiazole, in Ullman synthesis 115
Bromothiazole, with benzamide 48
Bromothiazole, with hydrazine 99
Brown root 138
Burmistrov method 128
Butylglycidylether, with azothiazoles 108
Cadmium complexes 104 156—168
Carbamates 241 242
Carbamates from azides 16
Carbamates from ethyl chloroformate 51. See also Thiazolyl carbamates
Carbamates with acetic anhydride 16
Carbamoyl chloride 431
Carbene intermediates 376 377
Carbinol see Alcohol
Carbinolamine intermediate 41
Carbocations 47
Carbocations from alcohols 39
Carbocations from diazoniums 108
Carbocations in acidic alkylations of aminothiazoles 38
Carbocations in F-C alkylations 80
Carbocations in nitrations 85
Carbon disulfide 60
Carbon disulfide in chemical structure determination 37
Carbon disulfide with 2-aminothiazole 60 94
Carbon disulfide with 2-iminothiazole 371
Carbon disulfide with 3-amino-2-iminothiazoline 131
Carbon disulfide with propargylamine 372
Carbonyl derivatives 40
Carbonyl derivatives with 2-aminopyridine 54
Carbonyl derivatives with aminothiazoles 40 54
Carboxylic acids see Acid (carboxylic)
Carcinolytic 152 153
Cardiovascular agent 138 441
Catalysis, by ammonium chloride 56
Catalysis, by diketones 53
Catalysis, by imidazole - 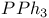 , mixture 90 , mixture 90
Catalysis, by nickel 116
Catalysis, by tin 116. See also Acid catalysis; Base catalysis
Catalysis, enzymatic 77
Catalysis, of acylation 53
Cationic dyes 105
Cellulose acetate 105
Cephalosporin structure 138
Charge control reactivity 118
Charge diagram,  -4-thiazoline-2-one 389 403 -4-thiazoline-2-one 389 403
Charge diagram,  -4-thiazoline-2-thione 389 -4-thiazoline-2-thione 389
Charge diagram, 2-hydroxythiazole 390
Charge diagram, 2-mercaptothiazole 390
Charge transfer, and U.V. spectra 21
Chelates, with metal ions 95
Chemical identification, of quaternarized aminothiazoles 37
Chemical identification, of sulfonyl thiazoles 75
Chemiluminescence, of firefly lanterns 421
Chemotherapeutic activity 152
Chichibabin reaction 12
Chichibabin reaction in synthesis of 2-aminothiazole 12
Chloraceiic acid, alkylation with 33
Chloraceiic acid, dialkylation with 37
Chloraceiyl chloride, with aminothiazoles 49
Chloracetic anhydride 124
Chloracetic ester, alkylation with 33
Chloride anion 121
Chlorination, by 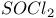 77 77
Chlorination, by HCl 77
Chlorination, of  -2-thiazoline-5-one 432 -2-thiazoline-5-one 432
Chlorination, of  -4-thiazoljne-2-one 402 -4-thiazoljne-2-one 402
Chlormethiazole, metabolism of 375
Chlorodiethylphosphate, reaction with,  -4-thiazolme-2-one 401 -4-thiazolme-2-one 401
Chlorodiethylthiophosphate, reaction with,  -4-thiazoline-2-one 401 -4-thiazoline-2-one 401
Chlorooxime 118
Chloroplatmate anion 121
Chlorothiazole, with  -hydroxyethylamine 34 -hydroxyethylamine 34
Chlorothiazole, with 2-imiro-4-thiazoline 57
Chlorothioformic phenyl ester, with 2-aminothiazoles 51
Chlorovinyl ketones, with 4-aminothiazoles 46
Chrysean, complexes with 122
Chrysean, synthesis of 16
CINDP 83
Circular dichroism,  -4-thiazoline-2-thione 380 -4-thiazoline-2-thione 380
cis-isomers 120
Claisen rearrangements, in 2-allyloxythiazole, kinetic siudy 410
Claisen rearrangements, stereochemistry 410
Cleavage, of 2-acetylimino-4-thiazolines 128
Cleavage, of aryl sulfonamidothiazole 117
CNDO calculations,  -4-thiazoline-thione-2, electronic transitions 380 -4-thiazoline-thione-2, electronic transitions 380
CNDO calculations,  -4-thiazoline-thione-2, PES spectra 381 -4-thiazoline-thione-2, PES spectra 381
CNDO calculations, charge diagram and PES spectra 17 18
Cobalt, complexes of 70 99 104 107 156 158 160 161
Coccidiostatic 152
Coding structures 139
Color-coupling agents 159
Colorimetry, in sulfathiazole determination 116
Complexes 57 99 70 104 107 108 119 120 122 154 156—168
Condensed heterocycles 33 34 42 43 44 45 49 50 53 54 56 57 58 60 62 65 69 78 95 96 130
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



 -4-thiazoline-2-one anion, examples
-4-thiazoline-2-one anion, examples 
 -
-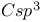 bond, in 3,4-diisopropyl-
bond, in 3,4-diisopropyl- ,
, 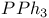 , mixture
, mixture 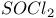
 -hydroxyethylamine
-hydroxyethylamine