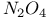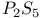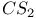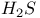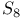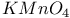 , oxidation by, 2-thiazolylsulfide 415 , oxidation by, 2-thiazolylsulfide 415
 , , 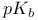 18 18
 , , 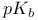 and amino-imino equilibrium 19 and amino-imino equilibrium 19
 , , 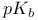 and substituents effects 19 91 and substituents effects 19 91
 , , 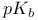 of of  -2-thiazoline-4-one, from UV experiments, from potentiometry 423 -2-thiazoline-4-one, from UV experiments, from potentiometry 423
 , , 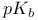 of of  -4-thiazoline-one 389 -4-thiazoline-one 389
 , , 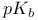 of 2-methoxy-4-methylthiazole 389 of 2-methoxy-4-methylthiazole 389
 , , 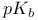 of 2-methylthiothiazole, in relation with nucleophilicity 405 of 2-methylthiothiazole, in relation with nucleophilicity 405
 , , 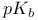 of 2-thiazolyl-oxides 409 of 2-thiazolyl-oxides 409
 , , 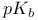 of acylaminothiazoles 91 of acylaminothiazoles 91
 , , 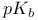 of azothiazoles 107 of azothiazoles 107
 , , 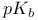 , correlation with sigma values 19 , correlation with sigma values 19
 , , 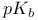 , representative values 20 , representative values 20
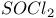 , chlorination with 77 , chlorination with 77
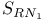 mechanism, and halothiazoles 18 mechanism, and halothiazoles 18
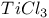 , reduction by 16 , reduction by 16
 -Chloracetamide, with acetamidothiazoles 33 -Chloracetamide, with acetamidothiazoles 33
 -Haloesters, reaction with, -Haloesters, reaction with,  -4-thiazolinc-2-thione anion 396 -4-thiazolinc-2-thione anion 396
 -Naphthol, coupling with 112 -Naphthol, coupling with 112
 -Adrenergic receptor, blocking agents 440 441 -Adrenergic receptor, blocking agents 440 441
 -Chloropropionyl chloride, with 2-hydrazinothiazoles 102 -Chloropropionyl chloride, with 2-hydrazinothiazoles 102
 -Chloropropionyl chloride, with aminothiazoles 50 -Chloropropionyl chloride, with aminothiazoles 50
 -Ketoesters, acylation with 53 -Ketoesters, acylation with 53
 -Ketoesters, with hydrazinothiazoles 102 -Ketoesters, with hydrazinothiazoles 102
 -Naphthol, coupling with 112 -Naphthol, coupling with 112
 -2-Oxazoline-5-one to -2-Oxazoline-5-one to  -2-thiazoline-5-one 429 -2-thiazoline-5-one 429
 -2-Oxazoline-5-one, cycloaddition reactivity 436 -2-Oxazoline-5-one, cycloaddition reactivity 436
 -2-Oxazoline-5-one, to 5-acetylthiothia-zole 417—418 -2-Oxazoline-5-one, to 5-acetylthiothia-zole 417—418
 -2-Thiazoline-4-one, -2-Thiazoline-4-one,  measurement 423 measurement 423
 -2-Thiazoline-4-one, -2-Thiazoline-4-one,  measurement, from potentiometry 423 measurement, from potentiometry 423
 -2-Thiazoline-4-one, -2-Thiazoline-4-one,  measurement, from UV data 423 measurement, from UV data 423
 -2-Thiazoline-4-one, 1,3-dipolar cyclo additions 425 426 -2-Thiazoline-4-one, 1,3-dipolar cyclo additions 425 426
 -2-Thiazoline-4-one, 1,3-dipolar cyclo additions, with dimethyl acetylene dicarboxylate 426 427 -2-Thiazoline-4-one, 1,3-dipolar cyclo additions, with dimethyl acetylene dicarboxylate 426 427
 -2-Thiazoline-4-one, 1,3-dipolar cyclo additions, with dimethyl fumarate 425 426 -2-Thiazoline-4-one, 1,3-dipolar cyclo additions, with dimethyl fumarate 425 426
 -2-Thiazoline-4-one, 1,3-dipolar cyclo additions, with dimethyl maleate 425 426 -2-Thiazoline-4-one, 1,3-dipolar cyclo additions, with dimethyl maleate 425 426
 -2-Thiazoline-4-one, 2-alkylthio derivatives, hydrolysis 423 -2-Thiazoline-4-one, 2-alkylthio derivatives, hydrolysis 423
 -2-Thiazoline-4-one, 2-alkylthio derivatives, reaction with amines 424 -2-Thiazoline-4-one, 2-alkylthio derivatives, reaction with amines 424
 -2-Thiazoline-4-one, 2-amino derivatives, reaction with secondary amines 424 -2-Thiazoline-4-one, 2-amino derivatives, reaction with secondary amines 424
 -2-Thiazoline-4-one, 2-chloro derivatives, hydrolysis of 423 -2-Thiazoline-4-one, 2-chloro derivatives, hydrolysis of 423
 -2-Thiazoline-4-one, 2-chloro derivatives, reaction with amines 424 -2-Thiazoline-4-one, 2-chloro derivatives, reaction with amines 424
 -2-Thiazoline-4-one, 2-methyl-5-substituted derivatives, reaction with -2-Thiazoline-4-one, 2-methyl-5-substituted derivatives, reaction with 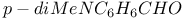 425 425
 -2-Thiazoline-4-one, 2-phenyl derivatives, dimers of 420 423 -2-Thiazoline-4-one, 2-phenyl derivatives, dimers of 420 423
 -2-Thiazoline-4-one, 2-phenyl derivatives, structure of 419 -2-Thiazoline-4-one, 2-phenyl derivatives, structure of 419
 -2-Thiazoline-4-one, 5-benzylidene derivatives from 425 -2-Thiazoline-4-one, 5-benzylidene derivatives from 425
 -2-Thiazoline-4-one, acylation 423 -2-Thiazoline-4-one, acylation 423
 -2-Thiazoline-4-one, dimerization of 2-phenyl derivatives 423 -2-Thiazoline-4-one, dimerization of 2-phenyl derivatives 423
 -2-Thiazoline-4-one, hydrolysis, of 2-alkylthio derivatives 423 424 -2-Thiazoline-4-one, hydrolysis, of 2-alkylthio derivatives 423 424
 -2-Thiazoline-4-one, hydrolysis, of 2-chloro derivatives 423 424 -2-Thiazoline-4-one, hydrolysis, of 2-chloro derivatives 423 424
 -2-Thiazoline-4-one, infrared 422 -2-Thiazoline-4-one, infrared 422
 -2-Thiazoline-4-one, methylation of 423 -2-Thiazoline-4-one, methylation of 423
 -2-Thiazoline-4-one, NMR of 422 -2-Thiazoline-4-one, NMR of 422
 -2-Thiazoline-4-one, nucleophilic reactivity at C-5, self condensation in relation with 425 -2-Thiazoline-4-one, nucleophilic reactivity at C-5, self condensation in relation with 425
 -2-Thiazoline-4-one, nucleophilic reactivity at C-5, with aryldiazonium salts 425 -2-Thiazoline-4-one, nucleophilic reactivity at C-5, with aryldiazonium salts 425
 -2-Thiazoline-4-one, nucleophilic reactivity at C-5, with arylnitroso derivatives 425 -2-Thiazoline-4-one, nucleophilic reactivity at C-5, with arylnitroso derivatives 425
 -2-Thiazoline-4-one, nucleophilic reactivity at C-5, with benzaldehyde 425 -2-Thiazoline-4-one, nucleophilic reactivity at C-5, with benzaldehyde 425
 -2-Thiazoline-4-one, nucleophilic reactivity at C-5, with benzoselenazolium salts 424 -2-Thiazoline-4-one, nucleophilic reactivity at C-5, with benzoselenazolium salts 424
 -2-Thiazoline-4-one, nucleophilic reactivity at C-5, with benzothiazolium salts 424 -2-Thiazoline-4-one, nucleophilic reactivity at C-5, with benzothiazolium salts 424
 -2-Thiazoline-4-one, nucleophilic reactivity of oxygen 423 424 -2-Thiazoline-4-one, nucleophilic reactivity of oxygen 423 424
 -2-Thiazoline-4-one, preparation of, from 4-aminothiazole 421 -2-Thiazoline-4-one, preparation of, from 4-aminothiazole 421
 -2-Thiazoline-4-one, preparation of, general 419 426 -2-Thiazoline-4-one, preparation of, general 419 426
 -2-Thiazoline-4-one, protomeric equilibrium 421 -2-Thiazoline-4-one, protomeric equilibrium 421
 -2-Thiazoline-4-one, protomeric equilibrium, infrared study of 421 -2-Thiazoline-4-one, protomeric equilibrium, infrared study of 421
 -2-Thiazoline-4-one, protomeric equilibrium, NMR study of 421 -2-Thiazoline-4-one, protomeric equilibrium, NMR study of 421
 -2-Thiazoline-4-one, protomeric equilibrium, solvent effect on 422 -2-Thiazoline-4-one, protomeric equilibrium, solvent effect on 422
 -2-Thiazoline-4-one, stability of 420 421 -2-Thiazoline-4-one, stability of 420 421
 -2-Thiazoline-4-one, syntheses, 2-alkylthio derivatives 419 -2-Thiazoline-4-one, syntheses, 2-alkylthio derivatives 419
 -2-Thiazoline-4-one, syntheses, 2-aryl-derivatives 419 420 -2-Thiazoline-4-one, syntheses, 2-aryl-derivatives 419 420
 -2-Thiazoline-4-one, syntheses, 2-arylthio derivatives 419 -2-Thiazoline-4-one, syntheses, 2-arylthio derivatives 419
 -2-Thiazoline-4-one, table of compounds 531—535 -2-Thiazoline-4-one, table of compounds 531—535
 -2-Thiazoline-4-one, to 2,4-thiazolinediones 423 424 -2-Thiazoline-4-one, to 2,4-thiazolinediones 423 424
 -2-Thiazoline-4-one, ultraviolet 422 -2-Thiazoline-4-one, ultraviolet 422
 -2-Thiazoline-4-thione 416 -2-Thiazoline-4-thione 416
 -2-Thiazoline-5-one, 2-benzylthio(oxo)-deiivatives, ring opening of 433 434 -2-Thiazoline-5-one, 2-benzylthio(oxo)-deiivatives, ring opening of 433 434
 -2-Thiazoline-5-one, 2-phenyl-4-substituted derivatives, ring opening 433 -2-Thiazoline-5-one, 2-phenyl-4-substituted derivatives, ring opening 433
 -2-Thiazoline-5-one, 4-phenylazo derivatives, rearrangement in thiazoline 435 -2-Thiazoline-5-one, 4-phenylazo derivatives, rearrangement in thiazoline 435
 -2-Thiazoline-5-one, 4-phenylazo derivatives, ring opening 434 -2-Thiazoline-5-one, 4-phenylazo derivatives, ring opening 434
 -2-Thiazoline-5-one, chloration of 432 -2-Thiazoline-5-one, chloration of 432
 -2-Thiazoline-5-one, cycloadditions reactions with, alkenes 436 -2-Thiazoline-5-one, cycloadditions reactions with, alkenes 436
 -2-Thiazoline-5-one, cycloadditions reactions with, alkynes 436 -2-Thiazoline-5-one, cycloadditions reactions with, alkynes 436
 -2-Thiazoline-5-one, dimerization catalyzed by -2-Thiazoline-5-one, dimerization catalyzed by 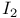 432 432
 -2-Thiazoline-5-one, enolisation with NaH 431 -2-Thiazoline-5-one, enolisation with NaH 431
 -2-Thiazoline-5-one, infrared data 430 -2-Thiazoline-5-one, infrared data 430
 -2-Thiazoline-5-one, mesoionic protomeric form 430 -2-Thiazoline-5-one, mesoionic protomeric form 430
 -2-Thiazoline-5-one, Michael addition of, 4-phenyl-1,2,4-triazolin-3,5-dione 433 -2-Thiazoline-5-one, Michael addition of, 4-phenyl-1,2,4-triazolin-3,5-dione 433
 -2-Thiazoline-5-one, Michael addition of, diethylazodicarboxylate 433 -2-Thiazoline-5-one, Michael addition of, diethylazodicarboxylate 433
 -2-Thiazoline-5-one, Michael addition of, maleic anhydride 433 -2-Thiazoline-5-one, Michael addition of, maleic anhydride 433
 -2-Thiazoline-5-one, NMR data 430 -2-Thiazoline-5-one, NMR data 430
 -2-Thiazoline-5-one, optically active 427 -2-Thiazoline-5-one, optically active 427
 -2-Thiazoline-5-one, preparation of 426 427 -2-Thiazoline-5-one, preparation of 426 427
 -2-Thiazoline-5-one, preparation of, from -2-Thiazoline-5-one, preparation of, from  -2-oxazoline-5-one 429 -2-oxazoline-5-one 429
 -2-Thiazoline-5-one, preparation of, from 4-alkylidene or 4-arylidene -2-Thiazoline-5-one, preparation of, from 4-alkylidene or 4-arylidene  -2-thiazoline-5-one 427 428 -2-thiazoline-5-one 427 428
 -2-Thiazoline-5-one, preparation of, from 5-alkoxythiazole 430 -2-Thiazoline-5-one, preparation of, from 5-alkoxythiazole 430
 -2-Thiazoline-5-one, preparation of, from N-thioacyl derivatives of amino acids 426 -2-Thiazoline-5-one, preparation of, from N-thioacyl derivatives of amino acids 426
 -2-Thiazoline-5-one, preparation of, optically active 427 -2-Thiazoline-5-one, preparation of, optically active 427
 -2-Thiazoline-5-one, protomeric equilibrium of, infrared 430 -2-Thiazoline-5-one, protomeric equilibrium of, infrared 430
 -2-Thiazoline-5-one, protomeric equilibrium of, NMR 430 -2-Thiazoline-5-one, protomeric equilibrium of, NMR 430
 -2-Thiazoline-5-one, protomeric equilibrium of, solvent effect on 430 -2-Thiazoline-5-one, protomeric equilibrium of, solvent effect on 430
 -2-Thiazoline-5-one, protomeric equilibrium of, substituent effect on 431 -2-Thiazoline-5-one, protomeric equilibrium of, substituent effect on 431
 -2-Thiazoline-5-one, protomeric equilibrium of, UV 430 431 -2-Thiazoline-5-one, protomeric equilibrium of, UV 430 431
 -2-Thiazoline-5-one, reaction with, acetyl chloride 431 -2-Thiazoline-5-one, reaction with, acetyl chloride 431
 -2-Thiazoline-5-one, reaction with, aldehyde 432 -2-Thiazoline-5-one, reaction with, aldehyde 432
 -2-Thiazoline-5-one, reaction with, benzoyl chloride 431 -2-Thiazoline-5-one, reaction with, benzoyl chloride 431
 -2-Thiazoline-5-one, reaction with, carbamoyl chloride 431 -2-Thiazoline-5-one, reaction with, carbamoyl chloride 431
 -2-Thiazoline-5-one, reaction with, enamines 432 433 -2-Thiazoline-5-one, reaction with, enamines 432 433
 -2-Thiazoline-5-one, reaction with, isocyanate 431 -2-Thiazoline-5-one, reaction with, isocyanate 431
 -2-Thiazoline-5-one, reaction with, ketone 432 -2-Thiazoline-5-one, reaction with, ketone 432
 -2-Thiazoline-5-one, reaction with, thioacetic acid 417 418 -2-Thiazoline-5-one, reaction with, thioacetic acid 417 418
 -2-Thiazoline-5-one, ring opening, 2-benzylthio(oxo)derivatives with, -2-Thiazoline-5-one, ring opening, 2-benzylthio(oxo)derivatives with, 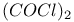 434 434
 -2-Thiazoline-5-one, ring opening, 2-benzylthio(oxo)derivatives with, amines 433 -2-Thiazoline-5-one, ring opening, 2-benzylthio(oxo)derivatives with, amines 433
 -2-Thiazoline-5-one, ring opening, 2-phenyl-4-substituted derivatives with, amino acids 433 -2-Thiazoline-5-one, ring opening, 2-phenyl-4-substituted derivatives with, amino acids 433
 -2-Thiazoline-5-one, ring opening, 2-phenyl-4-substituted derivatives with, hydrazine 433 -2-Thiazoline-5-one, ring opening, 2-phenyl-4-substituted derivatives with, hydrazine 433
 -2-Thiazoline-5-one, ring opening, 2-phenyl-4-substituted derivatives with, peptides 433 -2-Thiazoline-5-one, ring opening, 2-phenyl-4-substituted derivatives with, peptides 433
 -2-Thiazoline-5-one, ring opening, 4-phenylazo derivatives with amines 434 -2-Thiazoline-5-one, ring opening, 4-phenylazo derivatives with amines 434
 -2-Thiazoline-5-one, tables of compounds 526 527 -2-Thiazoline-5-one, tables of compounds 526 527
 -2-Thiazoline-5-one, transformation in triazoline derivatives 435 -2-Thiazoline-5-one, transformation in triazoline derivatives 435
 -2-Thiazoline-5-one, UV data 431 -2-Thiazoline-5-one, UV data 431
 -2-Thiazoline-5-thione, alkylation of, reaction with unsaturated compounds 417 -2-Thiazoline-5-thione, alkylation of, reaction with unsaturated compounds 417
 -2-Thiazoline-5-thione, preparation of, from 5-acetylthiothiazole 416 -2-Thiazoline-5-thione, preparation of, from 5-acetylthiothiazole 416
 -2-Thiazoline-5-thione, preparation of, from 5-thiocyanato thiazoles 416 -2-Thiazoline-5-thione, preparation of, from 5-thiocyanato thiazoles 416
 -2-Thiazoline-5-thione, preparation of, general 416 -2-Thiazoline-5-thione, preparation of, general 416
 -2-Thiazoline-5-thione, table of compounds 493—496 -2-Thiazoline-5-thione, table of compounds 493—496
 -2-Thiazoline-5-thione, tautomerism of 417 -2-Thiazoline-5-thione, tautomerism of 417
 -3-Thiazoline 86 87 -3-Thiazoline 86 87
 -4-Imidazoline-2-thione (diMel-3),methylation of 391 -4-Imidazoline-2-thione (diMel-3),methylation of 391
 -4-thiazoline-2-one 402 -4-thiazoline-2-one 402
 -4-Thiazoline-2-one by hydrolysis 130 -4-Thiazoline-2-one by hydrolysis 130
 -4-Thiazoline-2-one in bacterial culture medium 390 -4-Thiazoline-2-one in bacterial culture medium 390
 -4-Thiazoline-2-one, -4-Thiazoline-2-one,  389 389
 -4-Thiazoline-2-one, acylation of 402 -4-Thiazoline-2-one, acylation of 402
 -4-Thiazoline-2-one, ambident anion, behavior in -4-Thiazoline-2-one, ambident anion, behavior in 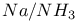 398 398
 -4-Thiazoline-2-one, ambidentreactivity 401 -4-Thiazoline-2-one, ambidentreactivity 401
 -4-Thiazoline-2-one, charge diagram, from HMO method 390 -4-Thiazoline-2-one, charge diagram, from HMO method 390
 -4-Thiazoline-2-one, charge diagram, from PPP method 389 390 -4-Thiazoline-2-one, charge diagram, from PPP method 389 390
 -4-Thiazoline-2-one, chlorination of 402 -4-Thiazoline-2-one, chlorination of 402
 -4-Thiazoline-2-one, dimerization of 403 -4-Thiazoline-2-one, dimerization of 403
 -4-Thiazoline-2-one, dipole moment, in relation with protomerism 389 -4-Thiazoline-2-one, dipole moment, in relation with protomerism 389
 -4-Thiazoline-2-one, electrophilic reactivity, in relation with PPP calculations 390 -4-Thiazoline-2-one, electrophilic reactivity, in relation with PPP calculations 390
 -4-Thiazoline-2-one, Friedel — Crafts reactions of 402 -4-Thiazoline-2-one, Friedel — Crafts reactions of 402
 -4-Thiazoline-2-one, Gattermann reaction on 402 -4-Thiazoline-2-one, Gattermann reaction on 402
 -4-Thiazoline-2-one, hydrolysis of 401 -4-Thiazoline-2-one, hydrolysis of 401
 -4-Thiazoline-2-one, infrared, in relation with protomerism 387 -4-Thiazoline-2-one, infrared, in relation with protomerism 387
 -4-Thiazoline-2-one, infrared, in relation with self association 389 390 -4-Thiazoline-2-one, infrared, in relation with self association 389 390
 -4-Thiazoline-2-one, infrared, representative data 390 -4-Thiazoline-2-one, infrared, representative data 390
 -4-Thiazoline-2-one, iodination 402 -4-Thiazoline-2-one, iodination 402
 -4-Thiazoline-2-one, Mannich reaction of 403 -4-Thiazoline-2-one, Mannich reaction of 403
 -4-Thiazoline-2-one, nitration of, kinetic of 402 403 -4-Thiazoline-2-one, nitration of, kinetic of 402 403
 -4-Thiazoline-2-one, nitration of, kinetic of, conformational analysis by 390 -4-Thiazoline-2-one, nitration of, kinetic of, conformational analysis by 390
 -4-Thiazoline-2-one, nitration of, kinetic of, in relation with protomerism 387 -4-Thiazoline-2-one, nitration of, kinetic of, in relation with protomerism 387
 -4-Thiazoline-2-one, nitration of, kinetic of, representative data 390 385 -4-Thiazoline-2-one, nitration of, kinetic of, representative data 390 385
 -4-Thiazoline-2-one, preparation of 372—377 -4-Thiazoline-2-one, preparation of 372—377
|  -4-Thiazoline-2-one, preparation of, by oxidation of -4-Thiazoline-2-one, preparation of, by oxidation of  -4-thiazoline-2-thione 374 397 -4-thiazoline-2-thione 374 397
 -4-Thiazoline-2-one, preparation of, from 2-biomo thiazolium salts 373 -4-Thiazoline-2-one, preparation of, from 2-biomo thiazolium salts 373
 -4-Thiazoline-2-one, preparation of, from 2-dim ethylamino thiazolium salts 373 -4-Thiazoline-2-one, preparation of, from 2-dim ethylamino thiazolium salts 373
 -4-Thiazoline-2-one, preparation of, from 2-imino- -4-Thiazoline-2-one, preparation of, from 2-imino- -4-thiazoline 374 -4-thiazoline 374
 -4-Thiazoline-2-one, preparation of, from 2-thiazole sulfonic acid 375 -4-Thiazoline-2-one, preparation of, from 2-thiazole sulfonic acid 375
 -4-Thiazoline-2-one, preparation of, from 2-thiazolyldiazonium salts 375 -4-Thiazoline-2-one, preparation of, from 2-thiazolyldiazonium salts 375
 -4-Thiazoline-2-one, preparation of, from 2-unsubstituted thiazolium salts 375 -4-Thiazoline-2-one, preparation of, from 2-unsubstituted thiazolium salts 375
 -4-Thiazoline-2-one, preparation of, from thiazolyl-2-oxide 409 410 -4-Thiazoline-2-one, preparation of, from thiazolyl-2-oxide 409 410
 -4-Thiazoline-2-one, protomeric equilibrium, dipole moment studies 389 -4-Thiazoline-2-one, protomeric equilibrium, dipole moment studies 389
 -4-Thiazoline-2-one, protomeric equilibrium, general 377 -4-Thiazoline-2-one, protomeric equilibrium, general 377
 -4-Thiazoline-2-one, protomeric equilibrium, infrared studies 387 -4-Thiazoline-2-one, protomeric equilibrium, infrared studies 387
 -4-Thiazoline-2-one, protomeric equilibrium, relation with self association 389 -4-Thiazoline-2-one, protomeric equilibrium, relation with self association 389
 -4-Thiazoline-2-one, protomeric equilibrium, solvent effect on 389 -4-Thiazoline-2-one, protomeric equilibrium, solvent effect on 389
 -4-Thiazoline-2-one, protomeric equilibrium, ultraviolet studies 387 -4-Thiazoline-2-one, protomeric equilibrium, ultraviolet studies 387
 -4-Thiazoline-2-one, reaction with, alkylating agents 401 -4-Thiazoline-2-one, reaction with, alkylating agents 401
 -4-Thiazoline-2-one, reaction with, chlorodiethylphosphate 401 -4-Thiazoline-2-one, reaction with, chlorodiethylphosphate 401
 -4-Thiazoline-2-one, reaction with, chlorodiethylthiophosphate 401 -4-Thiazoline-2-one, reaction with, chlorodiethylthiophosphate 401
 -4-Thiazoline-2-one, reaction with, diazonium salts 403 -4-Thiazoline-2-one, reaction with, diazonium salts 403
 -4-Thiazoline-2-one, reaction with, hydrazine 402 -4-Thiazoline-2-one, reaction with, hydrazine 402
 -4-Thiazoline-2-one, reaction with, phosphorus chloride 401 -4-Thiazoline-2-one, reaction with, phosphorus chloride 401
 -4-Thiazoline-2-one, reaction with, phosphorus pentasulfide 373 -4-Thiazoline-2-one, reaction with, phosphorus pentasulfide 373
 -4-Thiazoline-2-one, reaction with, phosphoryl chloride 401 -4-Thiazoline-2-one, reaction with, phosphoryl chloride 401
 -4-Thiazoline-2-one, reaction with, sulfenyl chloride 403 418 -4-Thiazoline-2-one, reaction with, sulfenyl chloride 403 418
 -4-Thiazoline-2-one, reduction of, with zinc dust 402 -4-Thiazoline-2-one, reduction of, with zinc dust 402
 -4-Thiazoline-2-one, Reimer — Tiemann reaction on 402 -4-Thiazoline-2-one, Reimer — Tiemann reaction on 402
 -4-Thiazoline-2-one, sel association, in relation with protomerism 377 -4-Thiazoline-2-one, sel association, in relation with protomerism 377
 -4-Thiazoline-2-one, sulfonation of 402 -4-Thiazoline-2-one, sulfonation of 402
 -4-Thiazoline-2-one, tables of compounds, 3,4,5-trisubstituted 520—523 -4-Thiazoline-2-one, tables of compounds, 3,4,5-trisubstituted 520—523
 -4-Thiazoline-2-one, tables of compounds, 3,4-disubstituted 519 -4-Thiazoline-2-one, tables of compounds, 3,4-disubstituted 519
 -4-Thiazoline-2-one, tables of compounds, 3,5-disubstituted 520 -4-Thiazoline-2-one, tables of compounds, 3,5-disubstituted 520
 -4-Thiazoline-2-one, tables of compounds, 3-pyrimidinylmethyl derivatives 523 524 -4-Thiazoline-2-one, tables of compounds, 3-pyrimidinylmethyl derivatives 523 524
 -4-Thiazoline-2-one, tables of compounds, 3-substituted 518 -4-Thiazoline-2-one, tables of compounds, 3-substituted 518
 -4-Thiazoline-2-one, tables of compounds, 4,5-disubstituted 515—518 -4-Thiazoline-2-one, tables of compounds, 4,5-disubstituted 515—518
 -4-Thiazoline-2-one, tables of compounds, 4-substituted 513 514 -4-Thiazoline-2-one, tables of compounds, 4-substituted 513 514
 -4-Thiazoline-2-one, tables of compounds, 5-substituted 513 -4-Thiazoline-2-one, tables of compounds, 5-substituted 513
 -4-Thiazoline-2-one, tables of compounds, miscellaneous 524 525 -4-Thiazoline-2-one, tables of compounds, miscellaneous 524 525
 -4-Thiazoline-2-one, to 2-imino-4-thiazolines 123 -4-Thiazoline-2-one, to 2-imino-4-thiazolines 123
 -4-Thiazoline-2-one, transformation in, 2-chlorothiazole 402 -4-Thiazoline-2-one, transformation in, 2-chlorothiazole 402
 -4-Thiazoline-2-one, transformation in, 2-chlorothiazole, thiochrome 402 -4-Thiazoline-2-one, transformation in, 2-chlorothiazole, thiochrome 402
 -4-Thiazoline-2-one, transformation in, 2-chlorotliiazoles 401 -4-Thiazoline-2-one, transformation in, 2-chlorotliiazoles 401
 -4-Thiazoline-2-one, transformation in, 2-chlorotliiazoles, 2-thiazolyloxides 401 -4-Thiazoline-2-one, transformation in, 2-chlorotliiazoles, 2-thiazolyloxides 401
 -4-Thiazoline-2-one, transformation in, 2-chlorotliiazoles, 2-unsubstituted thiazoles 402 -4-Thiazoline-2-one, transformation in, 2-chlorotliiazoles, 2-unsubstituted thiazoles 402
 -4-Thiazoline-2-one, transformation in, 2-chlorotliiazoles, N-substituted derivatives 401 -4-Thiazoline-2-one, transformation in, 2-chlorotliiazoles, N-substituted derivatives 401
 -4-Thiazoline-2-one, ultraviolet, in relation with protomerism 387 -4-Thiazoline-2-one, ultraviolet, in relation with protomerism 387
 -4-Thiazoline-2-one, ultraviolet, representative data 389 -4-Thiazoline-2-one, ultraviolet, representative data 389
 -4-Thiazoline-2-one, uses 438 -4-Thiazoline-2-one, uses 438
 -4-Thiazoline-2-selenone, photographic processes 370 -4-Thiazoline-2-selenone, photographic processes 370
 -4-Thiazoline-2-selenone, properties of 370 -4-Thiazoline-2-selenone, properties of 370
 -4-Thiazoline-2-selenone, silver halide emulsion 370 -4-Thiazoline-2-selenone, silver halide emulsion 370
 -4-Thiazoline-2-selenone, table of compounds 536 -4-Thiazoline-2-selenone, table of compounds 536
 -4-Thiazoline-2-thione 123 -4-Thiazoline-2-thione 123
 -4-Thiazoline-2-thione, ambident anion of, charge diagram from PPP calculation 390 -4-Thiazoline-2-thione, ambident anion of, charge diagram from PPP calculation 390
 -4-Thiazoline-2-thione, ambident anion of, reactivity 394 -4-Thiazoline-2-thione, ambident anion of, reactivity 394
 -4-Thiazoline-2-thione, ambident anion of, reactivity, in Mannich reaction 394 -4-Thiazoline-2-thione, ambident anion of, reactivity, in Mannich reaction 394
 -4-Thiazoline-2-thione, ambident anion of, reactivity, steric effect on 394 -4-Thiazoline-2-thione, ambident anion of, reactivity, steric effect on 394
 -4-Thiazoline-2-thione, ambident anion of, reactivity, with -4-Thiazoline-2-thione, ambident anion of, reactivity, with  -haloesters 396 -haloesters 396
 -4-Thiazoline-2-thione, ambident anion of, reactivity, with -4-Thiazoline-2-thione, ambident anion of, reactivity, with  -dimethylaminomethyl chloride 396 -dimethylaminomethyl chloride 396
 -4-Thiazoline-2-thione, ambident anion of, reactivity, with 1,4-dichloro-2-butene 396 -4-Thiazoline-2-thione, ambident anion of, reactivity, with 1,4-dichloro-2-butene 396
 -4-Thiazoline-2-thione, ambident anion of, reactivity, with 1,4-dichloro-2-butyne 396 -4-Thiazoline-2-thione, ambident anion of, reactivity, with 1,4-dichloro-2-butyne 396
 -4-Thiazoline-2-thione, ambident anion of, reactivity, with 2-chloro-propionitrile 396 -4-Thiazoline-2-thione, ambident anion of, reactivity, with 2-chloro-propionitrile 396
 -4-Thiazoline-2-thione, ambident anion of, reactivity, with acrylonitrile 394 -4-Thiazoline-2-thione, ambident anion of, reactivity, with acrylonitrile 394
 -4-Thiazoline-2-thione, ambident anion of, reactivity, with acyl chlorides 396 -4-Thiazoline-2-thione, ambident anion of, reactivity, with acyl chlorides 396
 -4-Thiazoline-2-thione, ambident anion of, reactivity, with alkyl halides 396 -4-Thiazoline-2-thione, ambident anion of, reactivity, with alkyl halides 396
 -4-Thiazoline-2-thione, ambident anion of, reactivity, with aryl halides 396 -4-Thiazoline-2-thione, ambident anion of, reactivity, with aryl halides 396
 -4-Thiazoline-2-thione, ambident anion of, reactivity, with cyanuric chloride 396 -4-Thiazoline-2-thione, ambident anion of, reactivity, with cyanuric chloride 396
 -4-Thiazoline-2-thione, ambident anion of, reactivity, with halogenothiazoles 396 -4-Thiazoline-2-thione, ambident anion of, reactivity, with halogenothiazoles 396
 -4-Thiazoline-2-thione, ambident anion of, reactivity, with lactones 396 -4-Thiazoline-2-thione, ambident anion of, reactivity, with lactones 396
 -4-Thiazoline-2-thione, ambident anion of, reactivity, with methylvinylketone 394 -4-Thiazoline-2-thione, ambident anion of, reactivity, with methylvinylketone 394
 -4-Thiazoline-2-thione, ambident anion of, reactivity, with N,N-dimethylthiocarbamoyl chloride 396 -4-Thiazoline-2-thione, ambident anion of, reactivity, with N,N-dimethylthiocarbamoyl chloride 396
 -4-Thiazoline-2-thione, ambident anion of, reactivity, with tetra-O-acetyl-D-glucopyranosyl bromide 394 -4-Thiazoline-2-thione, ambident anion of, reactivity, with tetra-O-acetyl-D-glucopyranosyl bromide 394
 -4-Thiazoline-2-thione, ambident anion of, reductive ring opening in -4-Thiazoline-2-thione, ambident anion of, reductive ring opening in 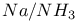 397 397
 -4-Thiazoline-2-thione, aryl derivatives, by isomerization reactions 373 -4-Thiazoline-2-thione, aryl derivatives, by isomerization reactions 373
 -4-Thiazoline-2-thione, charge diagrams, CNDO 385 -4-Thiazoline-2-thione, charge diagrams, CNDO 385
 -4-Thiazoline-2-thione, charge diagrams, PPP 385 389 -4-Thiazoline-2-thione, charge diagrams, PPP 385 389
 -4-Thiazoline-2-thione, circular dichroism 380 -4-Thiazoline-2-thione, circular dichroism 380
 -4-Thiazoline-2-thione, CNDO calculations 380 385 389 -4-Thiazoline-2-thione, CNDO calculations 380 385 389
 -4-Thiazoline-2-thione, complexes with, -4-Thiazoline-2-thione, complexes with, 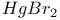 396 396
 -4-Thiazoline-2-thione, complexes with, Ag 386 -4-Thiazoline-2-thione, complexes with, Ag 386
 -4-Thiazoline-2-thione, complexes with, Cd 412 -4-Thiazoline-2-thione, complexes with, Cd 412
 -4-Thiazoline-2-thione, complexes with, Cu 412 386 -4-Thiazoline-2-thione, complexes with, Cu 412 386
 -4-Thiazoline-2-thione, complexes with, Mg 412 -4-Thiazoline-2-thione, complexes with, Mg 412
 -4-Thiazoline-2-thione, complexes with, Ni, Co 412 -4-Thiazoline-2-thione, complexes with, Ni, Co 412
 -4-Thiazoline-2-thione, complexes with, Pb 412 -4-Thiazoline-2-thione, complexes with, Pb 412
 -4-Thiazoline-2-thione, complexes with, transition metals 386 -4-Thiazoline-2-thione, complexes with, transition metals 386
 -4-Thiazoline-2-thione, complexes with, Zn 412 -4-Thiazoline-2-thione, complexes with, Zn 412
 -4-Thiazoline-2-thione, conformational analysis of alkyl substituents 385 -4-Thiazoline-2-thione, conformational analysis of alkyl substituents 385
 -4-Thiazoline-2-thione, desulfurization 393 397 399 -4-Thiazoline-2-thione, desulfurization 393 397 399
 -4-Thiazoline-2-thione, dipole moment, in relation with protomerism merism 379 -4-Thiazoline-2-thione, dipole moment, in relation with protomerism merism 379
 -4-Thiazoline-2-thione, dynamic NMR 384—385 -4-Thiazoline-2-thione, dynamic NMR 384—385
 -4-Thiazoline-2-thione, from 2-immo-4-thiazolihes 126 -4-Thiazoline-2-thione, from 2-immo-4-thiazolihes 126
 -4-Thiazoline-2-thione, hetero-association of, with 4-chlorophenol 377 -4-Thiazoline-2-thione, hetero-association of, with 4-chlorophenol 377
 -4-Thiazoline-2-thione, hetero-association of, with DMSO 377 -4-Thiazoline-2-thione, hetero-association of, with DMSO 377
 -4-Thiazoline-2-thione, infrared spectra 381 -4-Thiazoline-2-thione, infrared spectra 381
 -4-Thiazoline-2-thione, metal salts, asgennidices 412 -4-Thiazoline-2-thione, metal salts, asgennidices 412
 -4-Thiazoline-2-thione, metal salts, in analysis 412 -4-Thiazoline-2-thione, metal salts, in analysis 412
 -4-Thiazoline-2-thione, metal salts, in photographic processes 412 -4-Thiazoline-2-thione, metal salts, in photographic processes 412
 -4-Thiazoline-2-thione, metal salts, in rubber industry 412 -4-Thiazoline-2-thione, metal salts, in rubber industry 412
 -4-Thiazoline-2-thione, NMR, -4-Thiazoline-2-thione, NMR, 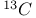 data 384 385 388 data 384 385 388
 -4-Thiazoline-2-thione, NMR, barrier to rotation of alkyl substituent 384 385 -4-Thiazoline-2-thione, NMR, barrier to rotation of alkyl substituent 384 385
 -4-Thiazoline-2-thione, NMR, representative data 384 -4-Thiazoline-2-thione, NMR, representative data 384
 -4-Thiazoline-2-thione, oxidation of, with -4-Thiazoline-2-thione, oxidation of, with 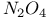 393 393
 -4-Thiazoline-2-thione, oxidation of, with ammonium persulfate 394 397 -4-Thiazoline-2-thione, oxidation of, with ammonium persulfate 394 397
 -4-Thiazoline-2-thione, oxidation of, with hydrogen peroxide 374 383 397 -4-Thiazoline-2-thione, oxidation of, with hydrogen peroxide 374 383 397
 -4-Thiazoline-2-thione, oxidation of, with iodine 393 -4-Thiazoline-2-thione, oxidation of, with iodine 393
 -4-Thiazoline-2-thione, oxidation of, with mercuric acetate 374 -4-Thiazoline-2-thione, oxidation of, with mercuric acetate 374
 -4-Thiazoline-2-thione, oxidation of, with NaOCl in presence of amine 411 -4-Thiazoline-2-thione, oxidation of, with NaOCl in presence of amine 411
 -4-Thiazoline-2-thione, oxidation of, with nitric acid andsodium nitrite 393 -4-Thiazoline-2-thione, oxidation of, with nitric acid andsodium nitrite 393
 -4-Thiazoline-2-thione, photoelectron spectra 381 -4-Thiazoline-2-thione, photoelectron spectra 381
 -4-Thiazoline-2-thione, PPP calculations 380 385 389 -4-Thiazoline-2-thione, PPP calculations 380 385 389
 -4-Thiazoline-2-thione, preparation of, by isomcrization of 5-methylenethiazoli-dine-2-thione 372 373 -4-Thiazoline-2-thione, preparation of, by isomcrization of 5-methylenethiazoli-dine-2-thione 372 373
 -4-Thiazoline-2-thione, preparation of, by rearrangement of thiazolyl-2-thioethers 406 -4-Thiazoline-2-thione, preparation of, by rearrangement of thiazolyl-2-thioethers 406
 -4-Thiazoline-2-thione, preparation of, from -4-Thiazoline-2-thione, preparation of, from  -4-thiazoline-2-one and -4-thiazoline-2-one and 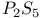 373 373
 -4-Thiazoline-2-thione, preparation of, from 1,3,4-thiadiazole-2-thione and alkyne 372 -4-Thiazoline-2-thione, preparation of, from 1,3,4-thiadiazole-2-thione and alkyne 372
 -4-Thiazoline-2-thione, preparation of, from 1,3-dithiolane and alkyne 372 -4-Thiazoline-2-thione, preparation of, from 1,3-dithiolane and alkyne 372
 -4-Thiazoline-2-thione, preparation of, from 1,3-dithiole-2-thione and amine 372 -4-Thiazoline-2-thione, preparation of, from 1,3-dithiole-2-thione and amine 372
 -4-Thiazoline-2-thione, preparation of, from 2-aminothiazole 370 -4-Thiazoline-2-thione, preparation of, from 2-aminothiazole 370
 -4-Thiazoline-2-thione, preparation of, from 2-benzylthiothiazolcs 405 -4-Thiazoline-2-thione, preparation of, from 2-benzylthiothiazolcs 405
 -4-Thiazoline-2-thione, preparation of, from 2-bromothiazole and thiourea 370 -4-Thiazoline-2-thione, preparation of, from 2-bromothiazole and thiourea 370
 -4-Thiazoline-2-thione, preparation of, from 2-iminothiazole and -4-Thiazoline-2-thione, preparation of, from 2-iminothiazole and 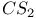 371 371
 -4-Thiazoline-2-thione, preparation of, from 2-iminothiazolium salts and -4-Thiazoline-2-thione, preparation of, from 2-iminothiazolium salts and 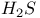 371 371
 -4-Thiazoline-2-thione, preparation of, from 2-thiazolyldiazonium salts and potassium -4-Thiazoline-2-thione, preparation of, from 2-thiazolyldiazonium salts and potassium  -4-Thiazoline-2-thione, preparation of, ethylxanthate or thiourea 370 -4-Thiazoline-2-thione, preparation of, ethylxanthate or thiourea 370
 -4-Thiazoline-2-thione, preparation of, from 2-thiocyanothiazole 370 -4-Thiazoline-2-thione, preparation of, from 2-thiocyanothiazole 370
 -4-Thiazoline-2-thione, preparation of, from 2-unsubstituted thiazoliumsalts 375 -4-Thiazoline-2-thione, preparation of, from 2-unsubstituted thiazoliumsalts 375
 -4-Thiazoline-2-thione, preparation of, from deoxybenzom and -4-Thiazoline-2-thione, preparation of, from deoxybenzom and 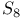 373 373
 -4-Thiazoline-2-thione, preparation of, from dithiazolylsulfidehydrochloride 405 -4-Thiazoline-2-thione, preparation of, from dithiazolylsulfidehydrochloride 405
 -4-Thiazoline-2-thione, preparation of, from thiazolyl-2-disulfides 412 -4-Thiazoline-2-thione, preparation of, from thiazolyl-2-disulfides 412
 -4-Thiazoline-2-thione, preparation of, propargyl amine and -4-Thiazoline-2-thione, preparation of, propargyl amine and 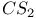 372 372
 -4-Thiazoline-2-thione, protomeric equilibrium, general 377 -4-Thiazoline-2-thione, protomeric equilibrium, general 377
 -4-Thiazoline-2-thione, protomerism, IR studies of 378 -4-Thiazoline-2-thione, protomerism, IR studies of 378
 -4-Thiazoline-2-thione, protomerism, RMN studies of 379 -4-Thiazoline-2-thione, protomerism, RMN studies of 379
 -4-Thiazoline-2-thione, protomerism, solvent effect on 379 -4-Thiazoline-2-thione, protomerism, solvent effect on 379
 -4-Thiazoline-2-thione, protomerism, ultraviolet studies 378 -4-Thiazoline-2-thione, protomerism, ultraviolet studies 378
 -4-Thiazoline-2-thione, Raman spectra 381 -4-Thiazoline-2-thione, Raman spectra 381
 -4-Thiazoline-2-thione, reaction with, alkylation agents, quantitative aspects, alcohols in -4-Thiazoline-2-thione, reaction with, alkylation agents, quantitative aspects, alcohols in  -4-Thiazoline-2-thione, reaction with, alkylation agents, quantitative aspects, acidic medium 392 -4-Thiazoline-2-thione, reaction with, alkylation agents, quantitative aspects, acidic medium 392
 -4-Thiazoline-2-thione, reaction with, alkylation agents, quantitative aspects, alkenes 392 -4-Thiazoline-2-thione, reaction with, alkylation agents, quantitative aspects, alkenes 392
 -4-Thiazoline-2-thione, reaction with, alkylation agents, quantitative aspects, alkyl halides 391 -4-Thiazoline-2-thione, reaction with, alkylation agents, quantitative aspects, alkyl halides 391
 -4-Thiazoline-2-thione, reaction with, alkylation agents, quantitative aspects, dihalogenoalkanes 391. See also Nucleophilic substitution -4-Thiazoline-2-thione, reaction with, alkylation agents, quantitative aspects, dihalogenoalkanes 391. See also Nucleophilic substitution
 -4-Thiazoline-2-thione, reaction with, mercury complexes 394 -4-Thiazoline-2-thione, reaction with, mercury complexes 394
 -4-Thiazoline-2-thione, reaction with, mercury complexes, tin complexes 394 -4-Thiazoline-2-thione, reaction with, mercury complexes, tin complexes 394
 -4-Thiazoline-2-thione, reactivity of, thiocarbonyl nudeophilicity 384 391—393 -4-Thiazoline-2-thione, reactivity of, thiocarbonyl nudeophilicity 384 391—393
 -4-Thiazoline-2-thione, rearrangement of 3-ammomethyl derivatives 400 -4-Thiazoline-2-thione, rearrangement of 3-ammomethyl derivatives 400
 -4-Thiazoline-2-thione, table of compounds, 3,4,5-trisubstituted 486—491 -4-Thiazoline-2-thione, table of compounds, 3,4,5-trisubstituted 486—491
 -4-Thiazoline-2-thione, table of compounds, 3,4-disubstituted 480—484 -4-Thiazoline-2-thione, table of compounds, 3,4-disubstituted 480—484
 -4-Thiazoline-2-thione, table of compounds, 3,5-disubstituted 485 -4-Thiazoline-2-thione, table of compounds, 3,5-disubstituted 485
 -4-Thiazoline-2-thione, table of compounds, 3-pyrimidinylmethyl derivatives 492 -4-Thiazoline-2-thione, table of compounds, 3-pyrimidinylmethyl derivatives 492
 -4-Thiazoline-2-thione, table of compounds, 3-substituted 479 480 -4-Thiazoline-2-thione, table of compounds, 3-substituted 479 480
 -4-Thiazoline-2-thione, table of compounds, 4,5-disubstituted 477—479 -4-Thiazoline-2-thione, table of compounds, 4,5-disubstituted 477—479
 -4-Thiazoline-2-thione, table of compounds, 4-substituted 474 475 -4-Thiazoline-2-thione, table of compounds, 4-substituted 474 475
 -4-Thiazoline-2-thione, table of compounds, 5-substituted 476 -4-Thiazoline-2-thione, table of compounds, 5-substituted 476
 -4-Thiazoline-2-thione, thin-layer chromatography 385 -4-Thiazoline-2-thione, thin-layer chromatography 385
|
 |
|
О проекте
|
|
О проекте



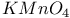 , oxidation by, 2-thiazolylsulfide
, oxidation by, 2-thiazolylsulfide  ,
, 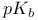
 -2-thiazoline-4-one, from UV experiments, from potentiometry
-2-thiazoline-4-one, from UV experiments, from potentiometry 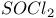 , chlorination with
, chlorination with 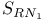 mechanism, and halothiazoles
mechanism, and halothiazoles 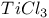 , reduction by
, reduction by  -Chloracetamide, with acetamidothiazoles
-Chloracetamide, with acetamidothiazoles  -Adrenergic receptor, blocking agents
-Adrenergic receptor, blocking agents 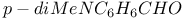
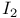
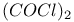
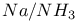
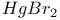
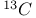 data
data