|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Metzger J.V. — Thiazole and Its derivatives (Part 2) |
|
|
 |
| Предметный указатель |
Hydrogen sulfide, with 2-bromo-4-methylthiazole 370
Hydrogen sulfide, with 2-iminothiazolium salts 371
Hydrogen sulfide, with 2-thiazolylcyanamides 94
Hydrogenation, with Ni catalyst 116
Hydrolysis, acidic conditions 92 99
Hydrolysis, of 2-dimethylaminothiazolium salts 373
Hydrolysis, of 2-halogenothiazolium salts 373
Hydrolysis, of 2-imino- -4-thiazoline 374 -4-thiazoline 374
Hydrolysis, of 2-thiazolylcyanamide 92
Hydrolysis, of 3-methyl- -4-thiazoline-2-one 401 -4-thiazoline-2-one 401
Hydrolysis, of 5-thiazolylthiouronium salts 516
Hydrolysis, of thiazolium salts 85
Hydrolysis, of thiazolylamidines 99
Hydrolysis, to sulfathiazoles 315
Hydroxyethylamine, with 2-chlorothiazole 34
Hydroxylamme 426
Hypnotic properties 138
Hypocholesterolemic 438
Hypoglycemic 440
Hypohalous acids 127
Hypotensive agent 146 147 149 153
Ignition 170
Imidazole, 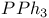 mixture 90 mixture 90
Imidazole, biheterocycle derivative 99
Imino-amino equilibrium see amino-imino
Indazole derivatives, by cyclization 47
Indicators 156—168
Indium complexes 157 158 160
Indolyl derivatives, with aminothiazoles 48
Infrared spectroscopy 23
Infrared spectroscopy and deuterated aminothiazoles 23
Infrared spectroscopy and dichroism 23
Infrared spectroscopy and Hammett constants 24
Infrared spectroscopy and isotopic substitution 23
Infrared spectroscopy and protomeric equilibria 23
Infrared spectroscopy and vibration-rotation bands 23
Infrared spectroscopy of  -2-thiazoline-4-ones, in relation with protomerism 422 -2-thiazoline-4-ones, in relation with protomerism 422
Infrared spectroscopy of  -4-thiazoline-2-one, in relation with protomerism 387 -4-thiazoline-2-one, in relation with protomerism 387
Infrared spectroscopy of  -4-thiazoline-2-one, in relation with self-association 390 -4-thiazoline-2-one, in relation with self-association 390
Infrared spectroscopy of  -4-thiazoline-2-one, representative data 390 -4-thiazoline-2-one, representative data 390
Infrared spectroscopy of  -4-thiazoline-2-thione 381 -4-thiazoline-2-thione 381
Infrared spectroscopy of 2-thiazolylsulfide 409
Infrared spectroscopy of 2-thiazolylsulfones 415
Infrared spectroscopy of 5-thiazolylsulfone 415
Infrared spectroscopy of azothiazoles 107
Infrared spectroscopy of complexes 104 120
Infrared spectroscopy of hydrazinothiazoles 105
Infrared spectroscopy of Schiff bases 41 99
Infrared spectroscopy of sulfonamidothiazoles 116
Infrared spectroscopy of sulfonylthiazoles 75
Infrared spectroscopy of thiazolyl thiourea 94 96
Infrared spectroscopy of thiazolylcarbamates 97
Infrared spectroscopy of thiazolylureas 93
Infrared spectroscopy, assignments in N-N-dialkylaminothiazoles 23
Infrared spectroscopy, frequencies and assignments 24
Infrared spectroscopy, protomerism studies 379
Inhibition of phosphatase 152
Insecticides 133 134 136 137 152 441 442
Intermediates in acylation reactions 47
Intermediates in acylation reactions in diazonium coupling 77
Intermediates in acylation reactions in mercuration 81
Intermediates in acylation reactions in N-oxides reactions 119
Intermediates in acylation reactions, carbinolamine as 41
Intermediates in acylation reactions, enamine-like 85
Intermediates in acylation reactions, formaldehyde Mannich base as 44
Intermediates in acylation reactions, hetaryne as 67
Intermediates in acylation reactions, nitramino 73
Intermediates in acylation reactions, open-chain 50 91
Intermediates in acylation reactions, perbromides 77
Intermediates in acylation reactions, tetrahedral 57. See also Amide ions; Anions; Carboca-tions; and Radical intermediates
Intestinal antiseptic 152
Intramolecular reactivity see Heterocyclizatione
Iodide 121
Iodine, as catalyst of rearrangement 406
Iodmation 77
Iodmation of  -4-thiazoline-2-one 402 -4-thiazoline-2-one 402
Ion pair mechanism, in reaction of benzene-thiolate with 2-halothiazole 404
Ion pairs, in ambident reactivity 40
Ionization potential, relation with nucleo-philic reactivity of  -4-thiazoline-2-thione 384 -4-thiazoline-2-thione 384
Iron 153
Iron, complexes 104 108 120 122
Iron, metabolism 152
Iron, reduction by 16 116
Isoamyl nitrite, in diazotizations 67
Isocyanate, Me 431
Isocyanate, phenyl 431
Isocyanates 58
Isocyanates to mesoionic compounds 65
Isocyanates to thiazolyl urea 58 92
Isocyanates with 2-imino-4-thiazolines 125
Isocyanates with hydrazinothiazoles 100 106
Isocyanates, acyl isocyanates 92
Isocyanates, alkyl isocyanates 92 118
Isocyanates, aryl versus alkyl 118
Isoindolenine derivative 56
Isomerism, E-Z equilibrium 23
Isomerization, catalyst in 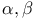 -unsaturated acids 438 -unsaturated acids 438
Isopropanol, with aminothiazoles 47
Isothiocyanates with 2-aminothiazoles 65
Isothiocyanates with azirines 15
Isothiocyanates with sulfathiazole 117
Isothiouronium salt 95
Isotopic labeling 370 410
Isotopic labeling and rearrangement 113
Isotopic labeling, 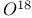 in firefly luciferin 421 in firefly luciferin 421
Ketone, reactions with,  -2-thiazoline-5-ones 432 -2-thiazoline-5-ones 432
Kinetic studies of ambident reactivities 63
Kinetic studies of Claisen rearrangement in 2-allyloxy-thiazole 410
Kinetic studies of nucleophilic attack 18
Kinetic studies, N-alkylation in 2-thiazolyl-thioethers 405
Kinetic studies, nitration of  -thiazoline-2-one 403 -thiazoline-2-one 403
Kinetic studies, of rearrangement 73 113
Kinetic studies, reaction of 2-halothiazoles, with alcohols 408—409
Kinetic studies, reaction of 2-halothiazoles, with mercaptans 405
Kinetic studies, S-alkylation in  -4-thiazoline-2-thione 384 391 392 -4-thiazoline-2-thione 384 391 392
Labeled compounds, 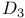 - - -4-thiazoline-2-thione, IR, Raman spectra 381 -4-thiazoline-2-thione, IR, Raman spectra 381
Lactames, reaction with,  -4-thiazoline-2-thione anion 396 -4-thiazoline-2-thione anion 396
Lead acetate 96
Lead dioxide, desulfurization with 98
Lead dioxide, oxidation by 45
Lead dioxide, radical with 101
Lead solutions 158
Leather 164
Leaving groups and diazonium salts 110
Leaving groups and sulfathiazole 117
Leaving groups, in acylations 47
Lettuces 136
Leucobase, oxidation of 45
Ligand in octahedral complex 108
Ligand, acetamidothiazoles as 70 120
Ligand, field effect 120 122
Linear free energy relationships 138
Lithium aluminum hydride, in reduction of Schiff bases 14
Lithium aluminum hydride, in reduction of thiazolines 127
Lithography printing plates 438
lminium salts, action of ammonia on 14
lsothiocyanates 58
lsothiocyanates with 2-aminobenzothiazoles 59
lsothiocyanates with 2-imino-4-thiazolines 58 125
m-Chloroperbenzoic acid, oxidation with 415
Magnesium sulfate 117
Magnetic moments 120
Magnetic susceptibilities, of 2-aminothiazole 29
Magnetic susceptibilities, of 2-imino-4-thiazolines 29
Magnetic susceptibilities, of Schiff bases 99
Maleic acid 40
Maleic anhydride 433
Malonic esters, acylation with 55
Malonyl chloride, acylation with 49
Mannich base as intermediate 44 91
| Mannich reaction 44
Mannich reaction of  -4-thiazoline-2-ones 403 -4-thiazoline-2-ones 403
Mannich reaction of  -4-thiazoline-2-thiones 394 -4-thiazoline-2-thiones 394
Mass spectra 27
Mass spectra of 2-aminothiazoles 27
Mass spectra of 2-cyclohexyldithiomethylthiazoles, in relation with vulcanization activity 412
Mass spectra of 2-hydrazinothiazoles 105
Mass spectra of acylaminothiazoles 91
Mass spectra of deuteroaminothiazoles 27
Mass spectra of Schiff bases 99
Mass spectra of thiazolyl-2-oxides 409
Mass spectra of thiazolylthioureas 94
Mass spectra, analogy with photochemistry 27
Mechanism, of electrophilic reactions 71 87 88
Mechanism, of halogenations 78
Mechanism, of Hantzsch reaction 122
Mechanism, of nitration 85
Mechanism, of nitrosation reactions 68
Mechanism, of rearrangement 73 82 83 95 113 130
Mechanism, of reduction 86 87
Mechanism, of retro-Hantzsch 84 102
Mercaptans, reaction with, 2-halothiazolcs 404
Mercaptans, reaction with, 5-bromothiazoles 418
Mercuration 81 129
Mercuric acetate 82 129
Mercuric acetate, reaction with  -4-thiazoline-2-thione 374 -4-thiazoline-2-thione 374
Mercuric bromide, complexes with  -4-thiazoline-2-thione 396 -4-thiazoline-2-thione 396
Mercuric halides 108
Mercuric oxide, with hydrazinothiazoles 100
Mercury 157 158 160
Mercury, complexes of 70 98 104 122
Mercury, complexes of, reaction with  -4-thiazoline-2-thiones 394 -4-thiazoline-2-thiones 394
Mesoionic hydroxythiazole, rearrangement of 375
Mesoionic protomer, in  -2-thiazoline-4-one 422 -2-thiazoline-4-one 422
Mesoionic xanthine, from alkylamino-thiazole 55 60 65
Metabolite, from 2-aminothiazole 42
Metabolite, in urine 85
Metabolite, radiolabeled 138
Metal complexes 154
Methoxy-4-methykhiazole, pKa in relation with protomerism 389
Methyl chloroformate 130
Methyl iodide, reactions with dialkyl-amino-thiazoles 32. See also Alkylation
Methylarmne 98
Methylarmne from 3-methyl- -4-thiazoline-2-one hydrolysis 401 -4-thiazoline-2-one hydrolysis 401
Methylation, of sulfathiazole 37. See also Alkylation
Methylvinylketone, reaction of, with ambident anion of  -4-thiazoline-2-thione 394 -4-thiazoline-2-thione 394
Michael addition, on  -2-thiazoline-5-one, 4-phenyl-1,2,4-triazolin-3,5-dione 433 -2-thiazoline-5-one, 4-phenyl-1,2,4-triazolin-3,5-dione 433
Michael addition, on  -2-thiazoline-5-one, with diethylazodicarboxylate 433 -2-thiazoline-5-one, with diethylazodicarboxylate 433
Michael addition, on  -2-thiazoline-5-one, with maleic anhydride 433 -2-thiazoline-5-one, with maleic anhydride 433
Microreversibility principle 85
Miticide 440
Mitodepressive 149 150
Mn complexes 104
Moessbauer studies 122
Molecular orbital calculations 17
Molecular orbital calculations and ambident reactivity 61 62
Molecular orbital calculations and amino-imino equilibrium 18
Molecular orbital calculations and electrophilic substitution 18
Molecular orbital calculations and HSAB 18
Molecular orbital calculations and nucleophilic substitution 18
Molybden complexes 98 155 158
Monoelectron transfer 409
Morphine tolerance inhibition 138
Muscle relaxant 150
Mutagenetic activity 153
Mycardiol  -stimulant 441 -stimulant 441
N,N-Dimethylthiocarbamoylchloride, reaction with,  -4-thiazoline-2-thione anion 396 -4-thiazoline-2-thione anion 396
N-bromosuccinimide, bromination with 77
Naphthalene, polarographic behavior 107
Narcotic 146
Nematocide 136 440
Neoplasm inhibitor 148
Nervous system action 138
Nickel 153 155 156 157 161
Nickel, catalyst 116
Nickel, complexes of 70 99 104 108 120
Niobium complexes 158
Niridazole 138
Nitraminothiazoles 112 113
Nitraminothiazoles by nitration 72 112
Nitraminothiazoles with diazomethane 37 112
Nitraminothiazoles, alkylation of 112
Nitraminothiazoles, nitration of 74
Nitraminothiazoles, rearrangement of 73 83 113
Nitrate anion 121
Nitration 72—75
Nitration in pyridine series 72
Nitration of  -4-thiazoline-2-one, kinetic investigations 402—403 -4-thiazoline-2-one, kinetic investigations 402—403
Nitration of 2-azidothiazoles 113
Nitration of 2-imino-4-thiazolines 125
Nitration of 2-thiazolyloxide (kinetics) 410
Nitration of 2-thiazolylsulfone 415
Nitration of 5;thiazolyloxide 436
Nitration of ammothiazoles 72
Nitration of heterocyclic molecules 71
Nitration, mechanism of 85
Nitric acid, in diazotation 65
Nitric acid, in nitration 72
Nitric acid, in nitrosation 65
Nitric acid, in oxidation 102 103
Nitrification inhibition 133 138
Nitro group, activation by 12
Nitro group, desactivation in diazotization 67
Nitro group, in antibacterial properties 136
Nitro group, in antiparasitic properties 136
Nitro group, reduction of 115
Nitrometry 116
Nitrosation 72—75
Nitrosation and diazotization 65 67
Nitrosation of 2-imino-4-thiazolines 125
Nitrosation of secondary amines 68
Nitrosation, mechanism of 68
Nitrosoimino-4-thiazoline 126
Nitrosulfonamides, oxidation of 115
Nitrous acid, with 2-hydrazinothiazoles 100
Nucleophilic additions, to double bonds 40
Nucleophilic substitution 417
Nucleophilic substitution and molecular orbital calculations 18
Nucleophilic substitution at oxygen in  -2-thiazoline-4-one 423 424 -2-thiazoline-4-one 423 424
Nucleophilic substitution by oxygen in  -2-thiazoline-5-one, with acetyl chloride 43 -2-thiazoline-5-one, with acetyl chloride 43
Nucleophilic substitution by oxygen in  -2-thiazoline-5-one, with benzoyl chloride 43 -2-thiazoline-5-one, with benzoyl chloride 43
Nucleophilic substitution by oxygen in  -2-thiazoline-5-one, with carbamoyl chloride 43 -2-thiazoline-5-one, with carbamoyl chloride 43
Nucleophilic substitution by oxygen in  -2-thiazoline-5-one, with isocyanate 43 -2-thiazoline-5-one, with isocyanate 43
Nucleophilic substitution on alcohol generated carbocations 39
Nucleophilic substitution on copper complex 12
Nucleophilic substitution on thiazolium salts 84
Nucleophilic substitution, activation by amino group of 18
Nucleophilic substitution, kinetic studies of 18
Nucleophilic substitution, nitrogen nucleophilicity in 2-thiazolyl-thioethers, kinetics 405
Nucleophilic substitution, sulfur nucleophilicity in  -4-thiazoline-2-thione, preparative aspects see Alkylation -4-thiazoline-2-thione, preparative aspects see Alkylation
Nucleophilic substitution, sulfur nucleophilicity in  -4-thiazoline-2-thione, quantitative aspects, Nucleophilic substitution, sulfur nucleophilicity in -4-thiazoline-2-thione, quantitative aspects, Nucleophilic substitution, sulfur nucleophilicity in  -4-thiazoline-2-thione, rate constants of 391 -4-thiazoline-2-thione, rate constants of 391
Nucleophilic substitution, sulfur nucleophilicity in  -4-thiazoline-2-thione, relation with ionization potential 384 -4-thiazoline-2-thione, relation with ionization potential 384
Nucleophilic substitution, sulfur nucleophilicity in  -4-thiazoline-2-thione, solvent effect on diazomethane reaction 395 -4-thiazoline-2-thione, solvent effect on diazomethane reaction 395
Nucleophilic substitution, sulfur nucleophilicity in  -4-thiazoline-2-thione, steric effect on 391 392 -4-thiazoline-2-thione, steric effect on 391 392
Nucleophilic substitution, sulfur nucleophilicity in  -4-thiazoline-2-thione, substituent effect on 391 392 -4-thiazoline-2-thione, substituent effect on 391 392
Nucleophilic substitution, sulfur nucleophilicity in  -4-thiazoline-2-thione, transition state of 391 392 -4-thiazoline-2-thione, transition state of 391 392
Nylon 163
Octahedral stereochemistry 120
Oleum, in nitration 72
Oleum, in sulfonation 75 113
Open-chain intermediates 91 102
Optical activity, in  -2-thiazoline-5-one 427 -2-thiazoline-5-one 427
Optical activity, in 2-aminothiazole derivatives 71
Oral bactericid 148
Oral schistosomicidel 140
Organometallics, in preparation of 2-hydrazinothiazoles 99
Osazones 100
Oscillopolography 116
Ouinazoline derivatives 123
Oxadiazole derivatives 1 32
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



 -4-thiazoline
-4-thiazoline 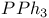 mixture
mixture 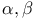 -unsaturated acids
-unsaturated acids 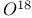 in firefly luciferin
in firefly luciferin 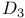 -
- -stimulant
-stimulant