|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Metzger J.V. — Thiazole and Its derivatives (Part 2) |
|
|
 |
| Предметный указатель |
Conformational analysis, of alkyl- -4-thiazoline-2-one 390 -4-thiazoline-2-one 390
Conformational analysis, of alkyl- -4-thiazoline-2-thione 385 -4-thiazoline-2-thione 385
Conformational analysis, of alkylamino-3-(2-thiazoloxy)-2-propanol 410
Conjugated acid, of 2-aminothiazoles 88
Conjugated base, of 2-aminothiazoles 88
Coordination in complexes 108 120
Copolymerization, butadiene 438
copper 156—168
Copper, chelates 95
Copper, complexes of 70 98 99 108 120
Copying material 169 171
Corrosion 170
Cosmetic resin 132
Coumarinyl derivatives 105
Coupling 76
Coupling with diazonium salts 76
Coupling, rate constants of 112
Crossover point 122
Cu powder, as catalyst 12
Culture protecting agent 135
Curie — Weiss law 121
Curtius degradation 90
Curtius rearrangement of azides 15 16
Cyanamides, with 4-aminothiazoles 58
Cyanide ligand 122
Cyanogen bromide 131
Cyanuric chloride, reaction with,  -4-thiazoline-2-thione anion 396 -4-thiazoline-2-thione anion 396
Cyclization see Heterocyclization
Cyclohexanol, with aminothiazoles 47
Cytostatic 134 150
Deactivation, by nitro group 67
Deamination, of 2-aminothiazoles 112
Decontracturing activity 147
Dehydrase of aminolevulinic acid 152
Desulfurization, of thiazolylthioureas 95 98
Desulfurization, with Al-Hg amalgam, of 2-thiazolyl-sulfide 405
Desulfurization, with Raney Ni, of  -4-tniazoline-2-thione 399 -4-tniazoline-2-thione 399
Desulfurization, with Zn powder, of 2-thiazolyl-sulfide 405
Deuterated compounds, IR spectra of aminothiazoles 23
Deuterated compounds, mass spectra of aminothiazoles 27
Deuterated compounds, sulfuric acid 92
Di-(trimethylsilyl) amine, reaction with  -4-thiazoline-2-thione 396 -4-thiazoline-2-thione 396
Dialkylaminocarbamoyl chlorides 126
Dialkylaminothiazoles, bromination of 78
Dialkylaminothiazoles, cleavage of 72
Dialkylaminothiazoles, IR spectra of 25
Dialkylaminothiazoles, mass spectra of 27
Dialkylaminothiazoles, nitration of 72 85
Dialkylaminothiazoles, rates constants with methyl iodide 32
Dialkylaminothiazoles, reduction of 87
Dialkylaminothiazoles, sulfonation of 75
Dialkylation, of 2-aminothiazole 37
Diaminothiazoles, quaternary salt 66
Diaminothiazoles, with benzaldehyde 47
Diaryl sulfonamides, cleavage of 117
Diazohydroxide, in diazotization 67
Diazomethane 37 112 116
Diazomethane, alkyl of,  -4-thiazoline-2-one 401 408 -4-thiazoline-2-one 401 408
Diazomethane, alkyl of,  -4-thiazoline-2-thione 395—396 -4-thiazoline-2-thione 395—396
Diazonium salts, and nitrosation 65
Diazonium salts, coupling with 76 108 110
Diazonium salts, in chemical structure determination 37
Diazonium salts, isolation of 66
Diazonium salts, of heterocyclic amines 65
Diazonium salts, solvent effects and 68
Diazonium salts, to hydrazinothiazoles 99
Diazonium salts, with  -4-thiazoline-2-ones 403 -4-thiazoline-2-ones 403
Diazonium salts, with azothiazoles 108
Diazonium salts, with iminothiazolines 128
Diazonium salts, with sulfathiazole 117
Dibrominated products 79
Dichroism, and infrared spectroscopy 23
Dicyandiamide, with 2-aminothiazole chlorhydrate 58
Dicyclohexyl carbodiimide 427
Diethylazodicarboxylate 433
Differential thermal analysis, on sulfathiazole 116
Dihalogenoalkanes, reaction with,  -4-thiazoline-2-thiones 291 -4-thiazoline-2-thiones 291
Diketones, with 4-aminothiazoles 46
Diketones, with hydrazinothiazoles 102
Dimerization, of  -4-thiazoline-2-one 403 -4-thiazoline-2-one 403
Dimerization, of 2-benzyloxy- -2-thiazoline-5-one 432 -2-thiazoline-5-one 432
Dimerization, of 2-phenyl- -2-thiazoline-4-one 419 423 -2-thiazoline-4-one 419 423
Dimethyl sulfate, alkylation with 93 112
Dimethyl sulfate, with 2-hydrazinothiazoles 106
Dimethyl sulfate, with sulfathiazoles 116
Dimethylacetylenedicarboxylate, reaction with,  -2-thiazoline-4-ones 426 -2-thiazoline-4-ones 426
Dimethylaminoethy] chloride, with 2-aminothiazole 35
Dimethylfumarate 417 425 426
Dimethylmaleate 426
Dimethylsulfoxide, hydrogen bonding, with  -4-thiazoline-2-thione 377 -4-thiazoline-2-thione 377
Dimethylsulfoxide, oxidation of thiazolium salts 376
Diorthohydroxazo 153
Dipole moment,  -4-thiazoline-2-one 389 -4-thiazoline-2-one 389
Dipole moment, 2-ammo thiazole 29
Dipole moment, 2-imino-4-thiazoline 29
Dipole moment, in  -4-thiazoline-2-thione, in relation with protomery 379 -4-thiazoline-2-thione, in relation with protomery 379
Disproportionate 107
Disulfide, in synthesis of 2-thiazolyl sulfide 404
Disulfonyl thiazoles, from 2-aminothiazoles 69 115 317
Dithiazolyl monosulfide (hydrochloride), to  -4-thiazoline-2-thione and 2-chlorothiazole 405 -4-thiazoline-2-thione and 2-chlorothiazole 405
Dithiazolyl monosulfide, preparation of, from 2-bromothiazole and thiourea 370
Dithiazolyl monosulfide, preparation of, from 2-halogeno thiazole and H: S 370
Dithiazolyl monosulfide, preparation of, from 2-halogeno thiazole and mercapto-thiazole 370
Dithiobiuret 85
Diuretic properties 150 152
Diuretic sulfonamide 414
DMF, in alkylation of aminothiazoles 35
DMF, in ambident reactivity 36
DMF, in cyclizations 50
DMSO, reaction with 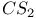 61 61
Dopamine content in brain 146
Dopamine hydroxylase inhibitor 152
Double bond, addition to 40
Double bond, ozonolysis of 71
DTAMR 4-(4,5-dimethyl-2-thiazolylazo)-2-methyl resorcinol 107 109
Dyes 56 77 105 108 132 153 154 156—168
Dynamic NMR, of 3,4-diisopropyl- -4-thiazoline-2-one 390 -4-thiazoline-2-one 390
Dynamic NMR, of N-substituted  -4-thiazoline-2-thione 384 387 388 -4-thiazoline-2-thione 384 387 388
Dysentry treatment 149
Echinochloa crus galli 135
Electronic circuits 438
Electronic transition, calculation of, in  -4-thiazoline-2-thione 380 381 -4-thiazoline-2-thione 380 381
Electronic transition, calculation of, in 2-methylthiothiazole 380
Electrophilic centers, ambidenf isothio-cyanate 126
Electrophilic centers, and HSAB 88
Electrophilic centers, substitution by aminothiazoles towards 32 39
Electrophilic substitution, and molecular orbital calculations 18
Electrophilic substitution, in  -4-thiazoline-2-one, relation with PPP calculations 390 -4-thiazoline-2-one, relation with PPP calculations 390
Electrophilic substitution, on  -4-thiazoline-2-thione 400 -4-thiazoline-2-thione 400
Electrophilic substitution, with amino-thiazole 71—84
Electrophoresis, of 2-aminothiazoles 30
Enamine, intermediate 85
Enamines, N-morpholino-1-cyclohexene, reaction with  -2-thiazoline-5-one 432 -2-thiazoline-5-one 432
Enamines, N-pyrrolidino-1-cyclohexene 432
Enamines, N-pyrrolidino-1-cyclohexene, reaction with  -2-thiazoline-5-one 432 -2-thiazoline-5-one 432
Enzymatic catalysis, in brommation 77 79
Enzyme activity 152
Epoxides see Ethylene oxide
EPR 83 101
Equilibrium, m acylations 47
Escherichia coli 139 140 152
ESR see EPR
Esters, acylation of aminothiazoles 47 53
Esters, with aminothiazoles 119
Ethoxy carbonylisothiocyanate 126
Ethyl aery late, acylation with 54
Ethyl chloroformate, to carbamate 51 96 97
Ethyl orthoformate, with  -2-thiazoline-5-one 432 -2-thiazoline-5-one 432
Ethyl orthoformate, with 3-amino-2-iminothiazoline 1 31
Ethylene diamine, in reaction with 2-halo-thiazoles 12
| Ethylene oxide, with aminothiazoles 34 38
Eutectic composition, in solid-state reactions 52
Exchange reactions, of hydrogen 85
Exchange reactions, with urea derivatives 93
Extraction, agents of metal ions 155
Extraction, of ammothiazole 30
Fatty acids 156—168
Fatty acids in octahedral complexes 108
Ferric chloride, oxidation with 102
Fezatione 439
Firefly decarboxy-ketoluciferin 420
Firefly lanterns, bioluminescence 421
Firefly lanterns, bioluminescence, mechanisms of 421
Firefly lanterns, lucioia cruaciata 421
Firefly lanterns, photinus pyralis 420
Firefly luciferin, chemiluminescence of 421
Firefly oxyluciferin 420
Firefly oxyluciferin, synthesis of 421
Firefly oxyluciferin, synthesis of, from oxidation of lucifeiin 421
Firefly oxyluciferin, synthesis of, in bioluminescence of firefly lanterns 421
Firefly oxyluciferin, synthesis of, in chemiluminescence of firefly luciferin 421
Fishes 42 43 138 146
Foam coating 170
Folic acid 40
Food flavoring 441
Formaldehyde 403
Formaldehyde in bisheterocyclization 45
Formaldehyde with sulfatiazole 117
Formaldehyde, Mannich base as intermediate 44
Formocibazol (sulfathiazole derivative) 117
Free radical see Radical intermediates
Friedel — Crafts acetylations 80
Friedel — Crafts acetylations, 4-alkylidene- -2-thiazoline-5-one 428 -2-thiazoline-5-one 428
Friedel — Crafts alkylations with alcohols 38 47 80
Frontier control reactivity 118
Fungicides 132 133 134 135 136 137 138 152 438 439 440 441 442
Furan, in diazotizations 67
Furan, in halogenation 78
Gallium complexes 156—168
Gas chromatography see Gas-liquid chromatography
Gas-liquid chromatography, 2-aminothiazoles determination by 30
Gastric acid secretion inhibitor 150
Gatterman reaction, of  -4-thiazoline-2-one 402 -4-thiazoline-2-one 402
Gear effect, in  -4-thiazoline-2-one 390 -4-thiazoline-2-one 390
Gear effect, in  -4-thiazoline-2-thione 384 -4-thiazoline-2-thione 384
Germanium complexes 156—168
Glucose, with sulfathiazoles 117
Glutathione 405
Glyoxylic acid, with sulfathiazole 117
Goitrogenic activity 153
Gold compounds 156—168
Gravimetric determination, of complexes 70
Grignard reagents, with 2-azidothiazoles 113
Grignard reagents, with 4 alkylidene- -2-thiazolme-5-one 428 429 -2-thiazolme-5-one 428 429
Grignard reagents, with azothiazoles 108 see
Grote's reagent, determination of ammothiazole 30
Growth enhancer 135 136
Guanidines 95 98 219.
H-NMR spectroscopy 25
H-NMR spectroscopy and amino-imino equilibria 25
H-NMR spectroscopy of  -2-thiazoline-4-ones, protomerism 422 -2-thiazoline-4-ones, protomerism 422
H-NMR spectroscopy of  -4-thiazoline-2-one, protomerism 389 -4-thiazoline-2-one, protomerism 389
H-NMR spectroscopy of  -4-thiazoline-2-one, representative data 385 390 -4-thiazoline-2-one, representative data 385 390
H-NMR spectroscopy of  -4-thiazoline-2-thione, protomerism 378 379 380 -4-thiazoline-2-thione, protomerism 378 379 380
H-NMR spectroscopy of  -4-thiazoline-2-thione, representative data 384 385 -4-thiazoline-2-thione, representative data 384 385
H-NMR spectroscopy of 2-thiazolyloxides 409
H-NMR spectroscopy of 2-thiazolylsulfides 404
H-NMR spectroscopy of 2-thiazolylsulfones 415
H-NMR spectroscopy of 4-thiazolyloxides 426
H-NMR spectroscopy of 5-thiazolyloxides 436
H-NMR spectroscopy of 5-thiazolylsulfides 418
H-NMR spectroscopy of 5-thiazolylsulfones 415
H-NMR spectroscopy of azothiazoles 105
H-NMR spectroscopy of thiazolyl carbamates 97
H-NMR spectroscopy, 2-aminothiazole representative shifts 26 107
H-NMR spectroscopy, 2-imino-4-thiazoline representative shifts 26
H-NMR spectroscopy, hindered rotation in 27
H-NMR spectroscopy, signal shape of protomeric forms 25
H-NMR spectroscopy, substituent effect in 25
Hair lotion 170
Halides, with azothiazoles 111
Halo thiazoles, 2-bromo-4-methylthiazole 370
Halo thiazoles, 2-bromothiazole 370
Halo thiazoles, 4-bromothiazole 426
Halo thiazoles, ammonolysis of 12
Halo thiazoles, and 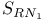 mechanism 18 mechanism 18
Halo thiazoles, by Sandmeyer reaction 12
Halo thiazoles, reaction with, ethylene diamine 12
Halo thiazoles, reaction with, rhodanine 79
Halo thiazoles, reaction with, thiocyanate 79
Halocarbonyl derivatives, with 2-aminothiazoles 33
Halogenation, of 2-aminothiazoles 77
Hammett constants and IR spectroscopy 24
Hammett constants, correlation with  19 19
Hansch equations 138
Hantzsch heterocyclization 115 122.
Hard acid or base see HSAB theory
Heat-sensitive 169
Heating curves, in solid-state reactions 52
Herbicide 133 134 135 136 438 439 440
Hetaryne, in diazotations 67
Heterocyclization of 2-imino-4-thiazoline 127
Heterocyclization of 2-imino-4-thiazoline, substituent effects on 62 63
Heterocyclizations 58 65 93 105 118
Heterocyclizations by Hantzsch method 115 122
Heterocyclizations of sulfenamido thiazoles 69
Heterocyclizations of thiazolyl ureas 37
Heterocyclizations to indazole derivatives 47
Heterocyclizations to Schiff bases 42
Heterocyclizations with 4-aminothiazole derivatives 46
Heterocyclizations, acid catalysed 91
Heterocyclizations, following acylation 49 50 53 54
Heterocyclizations, following halogenation 78 96
Hg see Mercury
Hindered rotation see Barrier to rotation
Histidine decarboxylase activity, inhibition of 438
HMO treatment, and amino-imino equilibrium 19 20
HMO treatment, and charge diagrams 17 18
HMO treatment, and protomeric equilibrium 2 100 377
HMO treatment, of  -4-thiazoline-2-thione 378 -4-thiazoline-2-thione 378
HMO treatment, of hydrazmothiazoles 100
Holmium complexes 158
HOMO (Higher Occupied Molecular Orbital) 61
Horticultural uses 134
HSAB theory, and ambident reactivity 5 61 62
HSAB theory, and ambident reactivity, of  -4-thiazoline-2 thione anion 397 -4-thiazoline-2 thione anion 397
HSAB theory, and electrophilic centers 88
HSAB theory, and molecular orbital calculations 18
HSAB theory, and stenc effects 63
HSAB theory, hard reagents 62 63 88
HSAB theory, soft reagents 62 63 88
HSAB theory, symbiotic behavior 63
hydration see Hydrolysis
Hydrazine 402 426 433
Hydrazines, dimaleic acid hydrazines 40
Hydrazines, on 2-bromothiazoles 99
Hydrazines, on 2-mercaptothiazoles 99
Hydrazines, with 2-hydrazinothiazoles 106
Hydrazines, with thiazolyl carbamates 97
Hydrides, in reductions 87 111
Hydrocarbon synthesis, from thiazolyl-2-thioethers 406
Hydrochloric acid 130
Hydrogen bond, 4-chlorophenol  -4-thiazoline-2 thione 377 -4-thiazoline-2 thione 377
Hydrogen bond, DMSO  -4-thiazoline-2-thione 377 see -4-thiazoline-2-thione 377 see
Hydrogen exchange 85
Hydrogen peroxide 92
Hydrogen peroxide, reaction with,  -4-thiazoline-2-thione 374 393 413 -4-thiazoline-2-thione 374 393 413
Hydrogen peroxide, reaction with,  -4-thiazoline-2-thione, 2-thiazolyl sulfide 405 -4-thiazoline-2-thione, 2-thiazolyl sulfide 405
Hydrogen sulfide, in synthesis of 5-aminothiazole 16
Hydrogen sulfide, with 2-amino-5-bromo-thiazole 417
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



 -4-thiazoline-2-one
-4-thiazoline-2-one 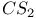
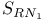 mechanism
mechanism 