|
|
 |
| јвторизаци€ |
|
|
 |
| ѕоиск по указател€м |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Israelachvili J.N. Ч Intermolecular and surface forces |
|
|
 |
| ѕредметный указатель |
Addivity 180 188 267
Addivity of van der Waals forces 89 106 177Ч179 202
Adhesion and surface energy 163 267 270 334 336Ч337
Adhesion of deformable surfaces 326 414Ч416
Adhesion of elastic bodies 326Ч327 336Ч337 414Ч416
Adhesion, bilayer 309 397 414Ч416 421
Adhesion, effect of adsorbed layers 207
Adhesion, effect of capillary forces 330Ч333 335
Adhesion, effect of meniscus 330Ч333 335
Adhesion, effect of solvation force 267Ч270
Adhesion, effect of surface asperities 323 329 333
Adhesion, effect of surface roughness 329 333
Adhesion, effect of tension 309
Adhesion, effect of water 210 271 333 336
Adhesion, energy 144 163 179 201 210 267Ч268 270 312Ч313
Adhesion, force 9Ч10 163 172 210 267 270 327 328 414Ч416
Adhesion, hysteresis 322Ч323
Adhesion, measurement 165Ч168 172 270 333 415
Adhesion, mechanics 326Ч329
Adhesion, membrane 397 414Ч416 421
Adhesion, specific 410Ч412
Adhesion, theoretical aspects 9Ч10 163 267Ч2.68 334 414Ч416
Adhesion, vesicle 414Ч416
Adhesion, work of 313
Adsorbed film 149Ч151 195 198 206 210
Adsorbed film of liquid 192Ч195 198 210
Adsorbed film, effect on adhesion 207 323
Adsorbed film, effect on contact angle 322Ч323
Adsorbed film, liquid helium 192Ч194
Adsorbed film, non-wetting 284
Adsorbed film, theoretical aspects 149Ч151 192Ч195
Adsorption on surfaces 147Ч151 195 206 236
aggregates see УAmphiphilic structuresФ УMicelleФ УSelf-assemblyФ
aging effects 303
Alder transition 93
Amphiphilic molecules 135 342Ч343
Amphiphilic molecules in biological membranes 342Ч343
Amphiphilic molecules, biological 342Ч343 387
Amphiphilic molecules, critical chain length 370
Amphiphilic molecules, exchange 346 375
Amphiphilic molecules, forces between 361 341
Amphiphilic molecules, lifetime in micelle 346 375Ч376
Amphiphilic molecules, lifetimes in aggregates 346 375
Amphiphilic molecules, melting temperatures 378
Amphiphilic molecules, optimal area 368Ч370
Amphiphilic molecules, packing of 370
Amphiphilic molecules, pK values 342
Amphiphilic molecules, self-assembly of 345
Amphiphilic molecules, structure 344
Amphiphilic molecules, unsaturated 342Ч343 387
Amphiphilic structures 344 346 371 380Ч381
Amphiphilic structures, bicontinuous 392
Amphiphilic structures, effect of temperature 380 382
Amphiphilic structures, effect of two chains 375
Amphiphilic structures, forces between 276 395 414Ч416
Amphiphilic structures, inverted 379 392 420
Amphiphilic structures, periodic 392
Amphiphilic structures, phase equilibrium 367
Amphiphilic structures, phase transisions 353
Amphiphilic structures, polydispersity 358Ч362 373Ч374 385
Amphiphilic structures, size distribution 354Ч362 373Ч374 385
Amphiphilic structures, transitions between 380Ч382 362Ч364 392
Amphoteric surfaces 236Ч238 280
Angle-averaged potential 60 61 66 74
Anisotropic interaction see УOrientation dependent interactionФ
ArchimedesТ Principle 145
Area of contact 143
Area of headgroups 380
Area of interaction 143 159 174
Area, effective, for spheres 143 174
Associated liquid 26 59Ч60 125Ч127 268
Association of molecules 133 139 141 142
Association of molecules and particles 133 139Ч142 145Ч146 151
Association of particles 141 142 145
Atmosphere, earthТs 21
Atmosphere, ionic see УDouble-layerФ
Atom-surface interaction 14Ч15 155Ч157 176Ч178
Atomic force microscopy (AFM) 10 14 15 173
Atomic size 110
Bare ion radius 55
Barometric distribution law 21
Bending modulus of membrane 383 308Ч309 382Ч385
Bernal Ч Fowler rules 286
Bilayer 344 375Ч378
Bilayer, diffusion in 376
Bilayer, dimensions 344 376
Bilayer, elasticity 308Ч309 377 382Ч385 421
Bilayer, flip-flop 376
Bilayer, fluid states of 37
Bilayer, fluidity of 383
Bilayer, forces between 199 226Ч228 252 256 257 276Ч277 284Ч285 304Ч309 311 395Ч399 403Ч409 419
Bilayer, free and supported 403
Bilayer, fusion 409 416Ч419
Bilayer, lipid diffusion 376
Bilayer, lipid dynamics in 377
Bilayer, solute diffusion across 344
Bilayer, structure 344 376
Bilayer-vesicle transition 364
Biocontinuous structures 392
Biological membrane 385Ч392
Biological membrane in cells 386
Biological membrane, composition 386
Biological membrane, fusion 409 416Ч419
Biological membrane, interactions 413
Biological membrane, properties 389
Biological membrane, structure 390Ч394 413
Bohr atom 84 88 179
Bohr radius 84
Boiling temperature and intermolecular forces 25Ч27 87 115Ч116
Boltzmann distribution 20Ч22 27 119 215
Boltzmann distribution of ions 231Ч233
Boltzmann factor 20Ч22 27
Boltzmann statistics 23
Boltzmann-averaged potential 60Ч62
Bond, chemical 31
Bond, covalent 31Ч32
Bond, directionality of 31Ч32
Bond, energy 19 32
Bond, hydrogen see УHydrogen bondФ
Bond, ionic 44 32Ч37
Bond, length 110Ч111
Bond, metallic 31Ч32
Bond, moment 49
Bond, physical 32
Bond, polarizability 69
Bond, strength 32
Born 160
Born energy 37Ч38 42Ч44 63Ч64 73Ч74
Born energy, limits of applicability 73Ч74
Born repulsion 109Ч112
Born, electrostatic 256Ч257
Born, entropic 407
Born, hard core 175
Born, head-group 256 311 399 405Ч408 414Ч416
Born, hydration 229 256Ч257
Born, solvation 145
Born, steric 160 175
Born, van der Waals 188Ч196 256
Boundary lipids 389
Bridging force between biopolymers 411Ч42
Bridging force of polymers 297 299Ч302 411Ч412
Bridging force, ionic 410
Brownian collisions 47 154Ч155
Brush, polymer 291
Brush, polymer, theory of 295
Bubble coalescence 281
Capacitor 34Ч35
Capillarity 5Ч8
Capillary, condensation 330
Capillary, effect on adhesion 330Ч333
| Capillary, forces 330 333
Capillary, rise, of liquids 5Ч8 229
Carbon 173
Casimir Polder equation 108
Cavity, energy 17 318
Cavity, nucleation of 286 334
Cellular membrane see УBiological membraneФ
Chain-melting temperature 378
Chaotropic agent 135
Charge density of surfaces 213Ч215
Charge electroneutrality 34
Charge regulation 230 235Ч236 243
Charge reversal 237
Charge-dipole interaction 50Ч64
Charge-fluctuation force 83
Chemical bond 32 46
chemical potential 20
Chemical potential in chemical equilibrium 20Ч22
Chemical potential in intermolecular interactions 20Ч22
Chemical potential in self-assembly 345
Chemical potential of gas 23
Chord theorem 143
Classification of forces 11Ч12 27Ч29
Clathrate 130
Clausius Ч Mossotti equation 79 82 102 123
Clays, forces between 226Ч228 276
Clays, hydration effects 226Ч227 276
Clays, surfaces of 276
Clays, swelling 172 226Ч227 279 281
Close packing 117
Close packing of colloidal particles 92Ч93
Close packing, nonspherical molecules 115Ч116
Close packing, random 120
Close packing, spherical molecules 92Ч93 120
Cluster 317 (see also УDropletФ)
Co-ions 214
Co-polymers 296
Co-surfactant 382
Coagulation 120 168
Coagulation, critical concentration of 247Ч248
Coagulation, rate 154Ч155
Cohesion, work of 313
Cohesive energy 18 26 143
Cohesive energy of atoms and molecules 18Ч20 25Ч26 86Ч89
Cohesive energy, molar 25Ч26 86Ч89
Colloid, order in 92 93 227
Colloidal particles, coagulation 154Ч155 247Ч250
Colloidal particles, DLVO forces between 247Ч250
Colloidal stability and instability 120 247Ч250 253 257 280 287 310Ч311 333
Combining relations 139 144 200
Computer simulation 44Ч447 220Ч221
Computer simulation of adsorbed polymers 292
Computer simulation of ions near surface 220Ч221 239Ч240
Computer simulation of liquids 265
Computer simulation of solvation forces 265Ч266
Constant charge interaction 245Ч246
Constant potential interaction 245Ч246
Contact angle 149Ч151 319 321Ч324 334Ч335 412
Contact angle of adhering vesicles 414Ч416
Contact angle, advancing and receding 321Ч324
Contact angle, hysteresis 321Ч324
Contact angle, low values 322
Contact angle, theoretical aspects 149Ч151 321 334
Contact area 159
Contact mechanics 326Ч329
Contact value theorem 11 225 265 308
Continuum theories 43
Continuum theories, limitations of 42Ч44
Cooperative interactions 127 318
Correlations in position 118
Coulomb, force 11 27Ч28 32Ч37 39 43Ч44
Coulomb, interaction 11 32Ч39 43Ч44 46 52 58 64 121 126
Coulomb, law 33
Counterion 214
covalent bond 31Ч32
creep 325
Critical aggregation concentration (CAC) 351Ч352 364
Critical coagulation concentration (CCC) 347Ч348
Critical micelle concentration (CMC) 348 351Ч352 375
Critical micelle concentration (CMC), effects on 355 392Ч393
Critical micelle concentration (CMC), increment per 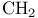 group 354Ч355 group 354Ч355
Critical micelle concentration (CMC), values for 355
Critical packing parameter 371Ч372
Critical point wetting 151
Curvature of surface 394
Curvature, elastic modulus of 382Ч385
de Broglie wavelength 23
Debye length 199 238 243
Debye length in van der Waals force 199
Debye unit 49
Debye Ч Hueckel equation 239
Debye Ч Hueckel theory 239
Debye Ч Langevin equation 70 79 82 97 123
Debye-induction interaction 75Ч76 94Ч95 182
Deformation, during adhesion, of particles 326Ч329
Deformation, during adhesion, of vesicles 414Ч416
Density distribution in liquids 18Ч19 117Ч119 261Ч264
Density distribution of molecules 18Ч19 261Ч264
Density distribution of polymer layer 292
Density distribution, function 19 117Ч118
Depletion force 302Ч303 310Ч311
Derjaguin approximation 161Ч164 172 175 243 270 326
Derjaguin Ч Landau Ч Verwey Ч Overbeek (DLVO) theory 246Ч250 (see also УDLVO forcesФ)
Derjaguin Ч Landau Ч Verwey Ч Overbeek (DLVO) theory, limitations 252Ч254
Desorption 147Ч151
Detergent action 7 336
dielectric constant 29 41 44 79Ч80
Dielectric constant, limitation at small distances 42Ч44 79Ч82
Dielectric constant, role in interactions 42Ч44 79Ч81
Dielectric constant, temperature-dependence of 80Ч81
Dielectric constant, values 41
Dielectric sphere in medium 77Ч81
Diffuse double-layer see УDouble-layerФ
Diffuse interface 92 288
Dipolar interactions 50Ч64 128
Dipolar ions 40 48
Dipolar molecule see УPolar moleculeФ
Dipole 27 48
Dipole moment 48Ч49
Dipole moment of bond 49
Dipole moment, water 37
Dipole, effect on forces 273
Dipole, field 74 254Ч256
Dipole, forces between 256
Dipole, induced 67Ч73 84
Dipole, interactions 50Ч64 128
Dipole, lattice 254Ч256
Dipole, permanent 48Ч49
Dipole, rotating 80 82
Dipole, self-energy 50
Dipole, surface 254Ч256
Dipole-dipole interaction 57Ч66 128 256
Directionality of bonds 127Ч128
Directionality of van der Waals force 89 115
Discrete charge effects 254Ч256
Disjoining pressure 192Ч194 229
Dispersion interaction 83Ч89 (see also УVan der Waals interactionФ)
Distribution function 19
Distribution function, density 117Ч118
Distribution function, radial 117Ч118
Divalent ion effects 228
Divalent ion effects on forces 257 397
Divalent ion effects on surface charge and potential 237
DLVO forces 246Ч253 277
DLVO forces between bilayers 256 397Ч398
DLVO forces in colloidal stability 247Ч250
DLVO forces in non-aqueous liquids 273Ч274
DLVO forces, measurements 250Ч253 397
DLVO theory see УDerjaguin Ч Landau Ч Verwey Ч Overbeek theoryФ
Double-layer 214
|
|
 |
| –еклама |
 |
|
|
 |
|
ќ проекте
|
|
ќ проекте



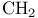 group
group