јвторизаци€
ѕоиск по указател€м
Farges J. (ed.) Ч Organic conductors: fundamentals and applications
ќбсудите книгу на научном форуме Ќашли опечатку?
Ќазвание: Organic conductors: fundamentals and applicationsјвтор: Farges J. (ed.) јннотаци€: Discussing the key issues that distinguish organic conductors from more conventional conductive compounds, this comprehensive and up-to-date reference examines all aspects of organic conductors;detailing the most recent theoretical concepts and current laboratory methods of synthesis, measurement, control, and analysis. Describes the latest advances in molecular-scale engineering, including switching and memory systems, Schottky and electroluminescent diodes, field-effect transistors, and photovoltaic devices and solar cells! Focusing on basic chemical and physical properties, Organic Conductors covers the theory of low-dimensional conductors delineates how organic conductors fit in with the rest of condensed-matter physics investigates the molecular design and synthesis of organic conductors analyzes techniques to characterize organic conductors such as crystallographic measurements and electronic and molecular spectroscopies reviews the magnetic electron spin resonance and nuclear magnetic resonance properties or organic conductors explores the physical properties of organic conductors such as electronic instabilities and phase transitions considers the potential application of organic materials and polymers in electronics provides a summary of organic superconductors, including fullerenes and more!
язык: –убрика: ‘изика /‘изика твЄрдого тела /ѕриложени€ /—татус предметного указател€: √отов указатель с номерами страниц ed2k: ed2k stats √од издани€: 1994 оличество страниц: 854ƒобавлена в каталог: 21.08.2005ќперации: ѕоложить на полку |
—копировать ссылку дл€ форума | —копировать ID
ѕредметный указатель
Polymers, solubility 503Ч505 541 569 613 Polymers, solubility, acquired on substitution 557 Polymers, stability 526 559 Polymers, stretching 542Ч569 Polymers, stretching with a plasticizer 552 565 566 Polymers, stretching, oriented samples 547 564Ч570 598 627 649 Polymers, substituents 520 531 557Ч564 570 596 631 Polymers, substituents, side chains, side groups 520 557Ч564 633 Polymethylmethacrylate 633 Polyoctylthiophene (POT) 562 569 Polyparaphenylene (PPP) 502 522 529 540 551 571 581 582 591 604 Polyphenylenevinylene (PPV) 502 513 517Ч519 551Ч631 Polyphenylenevinylene (PPV), substituted 563Ч564 615 627 Polypyrrole (PPy) 527Ч542 615 648 676Ч678 polystyrene 633 Polystyrene-polyacetylene (PS-PA) 569 Polythiophenes (PT) 502 513Ч529 542 551 574 591Ч613 677 679 769 Polythiophenes (PT), substituted 504 560 568 600 Polythiophenes (PT), substituted, poly(3-alkylthiophene) (P3AT) 558Ч566 624 Polyvinylcarbazole (PVK) 504 528 798 816 Porphyrins 695 717Ч718 815 Power law dependences 35Ч66 361Ч370 375 415Ч436 666 765 Pressure effects 16Ч17 63 64 341Ч343 365Ч387 418Ч475 773Ч774 Proteins 153 707 720Ч723 Purines 695 711 714Ч717 PVK:TNF 799 Pyranthrone 4 Pyranylidenes 82 Pyrazolines 805 Pyrene 304 694 Pyrimidines 711 714Ч717 Pyrimidium 777 Pyrrole 497 502 527 769 Quantum chemistry 418 500 501 513 514 661 676 Quantum chemistry, ab initio methods 197 592 Quantum chemistry, ab initio methods, HF-SCF 592 Quantum chemistry, semiempirical methods, Hueckel 197 507 513 592 Quantum chemistry, semiempirical methods, MNDO 592 Quantum chemistry, semiempirical methods, Valence effective Hamiltonian (VEH) 515 592Ч597 604 Quasi-particles 33Ч40 230 472 511Ч518 587 Quaterphenyle 556 Quinacridones 816 Quinolinium see " Quiquethiophene/isocaic acid LB films 776 Random phase approximation (RPA) 39Ч62 Random walk theory 664 Reciprocal space 151 154 187 188 207 542 Relaxation rate/time 230 244 365Ч367 377 388 623 Renormalization group (RG) method 36Ч64 414 415 Resonance valence bond (RVB) 61 Response function 33Ч64 373 410Ч416 Retinol/iodine complex 710 Rhodamines 816 818 RNA (ribonucleic acid) 713 716 719 Ruderman Ч Kittel interaction 273 S,S-Dimethyl-bis(ethylenedithio)tetrathiafulvane (S,S-DMBEDT-TTF) 125 S-methylthiouronium salt 252 Salicylic acid 699 Scaling 36Ч41 Scattering 30 53Ч54 288 365 372 394 659 662 see Scattering, backward/forward 30 44 53Ч54 256 281 282 289 304 379 409 411 433 Scattering, diffusive/nondiffusive 282 304 390 Scattering, elastic/inelastic 378 379 396 446 617 Scattering, interchain/intrachain 30 278 434 Scattering, mechanisms 368 369 375 Scattering, rate, time 368Ч370 377 396 483 Scattering, umklapp 30Ч63 331 336 369 411 433 Semimetallic state 365 380 387 442 502 Semimetallic state, two dimensional 444 474Ч478 Serotonin 718Ч721 Shubnikov Ч de Haas method 17 197 Solid-solid charge-transfer reactions 349Ч351 Solitons 8 26 47Ч55 65 373 503Ч516 541 570 592 671 674 683 698 Solitons, charged/neutral 51 55 511 575Ч609 658Ч676 Solitons, defect-like 340 342 Solitons, domain wall 511 670 Solitons, motion 340 511 548 669 671 Solitons, soliton-antisoliton pair 65 513 514 674 684 Solvation 6 Solvents 6 19 84 135 139 302 305 504 541 559Ч568 650 655 696Ч707 760 762 769 801 803 Specific heat 182 387 446 Spin 214 215 272 278 669Ч684 Spin density wave 13Ч17 33Ч66 204 263 283Ч286 365Ч396 411Ч465 469 474 Spin density wave 463 469Ч472 Spin density wave 468 Spin density wave 472 Spin density wave 472Ч474 Spin density wave 17 64Ч65 366 387 390 444 474Ч487 Spin density wave 481Ч484 Spin density wave 64 365 463Ч475 487 Spin density wave 465Ч468 Spin density wave 427 465 473 Spin density wave 461 465Ч473 Spin density wave 469 Spin density wave 393 462 468 Spin density wave 475 Spin density wave 469 Spin density wave 468 Spin flop transition 365 463 Spin susceptibility 34 35 42Ч43 54 63 269Ч309 369Ч380 387 420Ч423 431Ч433 458 463 481 517 518 523 666Ч684 692 764 767 see Spin susceptibility, box model 677Ч679 Spin susceptibility, Curie law 273 297 302 305 675 Spin susceptibility, Curie law versus Pauli law 679Ч682 Spin susceptibility, Curie Ч Weiss law 273 275 295Ч297 301 328 Spin susceptibility, diamagnetic susceptibility (Landau Ч Peierls) 270 271 288 371 387 Spin susceptibility, enhanced susceptibility 274 286 293Ч296 330 373 458 670 Spin susceptibility, enhanced susceptibility, Stoner-type enhancement factor 43 60 274 Spin susceptibility, Lande factor 272 273 Spin susceptibility, Pauli law 34 60 271 274 281 286 293 429 459 503 666 675 Spin susceptibility, singlet-triplet 64 215 276 297 299 327 Spin susceptibility, two-chain systems 281 283 286Ч289 Spin, concentration 511 513 669Ч679 Spin, doping produced 674Ч684 Spin, glass 54 681 Spin, motion 282 669 672 680 Spin, wave 13 272 Spinons 34 35 Squaraines 803 805 Squarylium dyes 803 815 Starch/iodine complex 711 Steric effects 94 103 331Ч336 551 558 Stilbene derivatives 805 Structural transitions see "Phase transitions" Su Ч Schrieffer Ч Heeger (SSH) Hamiltonian 31 43 44 48 49 53 507Ч512 520 549 575 577 589 590 671 Symmetry, broken symmetry 26 27 463 Symmetry, crystal symmetry 177 368 Symmetry, crystal symmetry and Neumann's principle 177 Synthetic metals 685 T-J model 32 43 61 64 67 TCNE 694 TCNQ 5 18 84 132 232 242 350Ч351 710 762Ч768 778 TCNQ salts 132 162 191 195 200 212 237Ч242 252Ч261 360 365 392 762Ч769 783 TCNQ, derivatives 93 94 103 108 766 768 Tetraalkylammonium salts 134 Tetrachalcogenafulvalenes 82 118 125 Tetrachalcogenapolyacenes 132 Tetrachalcogenotetracenes 85 Tetracyano-p-benzoquinone 93 Tetracyanodiphenoquinodimethane 94 Tetracyanoethylene (TCNE) 93 298 Tetracyanotetrahydropyrenoquinodimethane 94 Tetracycline 698 Tetraethylammonium 248 Tetrafluorotetracyanoquinodimethane 133 Tetrahalotetrathiafulvalene 124 Tetrahydrol,2,7,8-dicyclopentaperylene 305 Tetrakis(alkylthio)tetrathiafulvalenes 100 Tetrakis(methyltelluro)tetrathiafulvalenes 100 343 Tetramethyl-porphyrin complexes 707 Tetramethylammonium cation 99 Tetramethyltetraselenafulvalene see "TMTSF" Tetramethyltetrathiafulvalene see "TMTTF" Tetraselenafulvalene (TSF) 118 126 129 Tetraselenatetracene 132 Tetrasubstituted ethylenes 86 Tetratellurafulvalene (TTeF) 100 118 131 132 Tetrathiafulvalene see "TTF" Tetrathianaphthalene (TTN) 85 Tetrathiatetracene (TTT) 132 352 Thermal expansion 148 149 175Ч177 286 375 545 Thermal hysteresis 259 Thermal losses 345 Thermal motion 150 203 588 692 Thermionic emission 606 Thermopower 330 337 350Ч351 439 598 666 764 772 773 Thiapendione 121 Thiapyrilium salt 803 Thin-films 230 396 397 see Thiophene 500 513 561 562 622 631 TMA-TCNQ-I 244 TMTSF 129 140 161 210 370 396 406 TMTSF-DMTCNQ 289 352Ч353 380 394 TMTTF 396 766Ч768 TMTTF-TCNQ 373 Tranquilizers 705 708 721 Transfer (overlap) integrals 28 29 149 163 164 197 198 209 211 244 245 261 274 299 366 418 422 454 495 507 509 513 519 659 Transfer (overlap) integrals, interchain/intersheet 209 278 366 388 406 418 484 518 591 648 662 Transition metal complexes, LB films 769Ч771 Transition metal complexes, metal-dithiolate complexes 769 Transport properties see "Electrical properties" traps 580 598Ч625 651 674 797Ч818 Tri(tetrathiafulvalenyl)phosphine 91 Triarylamines 805 Triarylmethanes 805 Triethylammonium see "TEA(TCNQ)_{2}" Trimethylammonium-TCNQ-I (TMA-TCNQ-I) 155Ч158 181 182 188 200Ч202 208 Triphenylmethane dyes 818 TSeF-TCNQ 373 380 TSF 90 TTeF 90 TTF 5 18 84Ч100 118 123 124 134 251 337 350Ч351 359 360 392 768 TTF salts 240 242 250 257 TTF, derivatives 86Ч100 118Ч125 TTF, giant analogs 250Ч251 TTF-Cl 240 TTF-p-chloranil 341 342 TTF-TCNQ 5 10 29 46 65 97 98 132 136 155Ч217 237Ч259 287 289 338Ч339 350 359Ч393 463 488 767 TTF-TCNQ composites 350Ч351 Tunneling 10 28 47Ч49 55Ч57 66 394 598 606 607 619Ч625 664 668 698 see Tunneling, two-particle 56 57 66 Vibronic or e-mv coupling 230Ч260 369 391Ч393 454 573 587 591 597 see Vibronic or e-mv coupling, microscopic theory 230Ч235 247Ч252 260 Violanthrene-iodine complex 694 Violanthrone 4 Vitamins 708Ч711 Vitamins, vitamin A (retinol) 710 Vitamins, vitamin C (ascorbic acid) 711 722 Vitamins, vitamin K 711 Vitamins, vitamins B 710Ч711 Weiss field 272 XY model 413 Yukawa potential 14
–еклама
 |
|
ќ проекте
|
|
ќ проекте



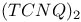
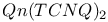 "
"
 (SDW)
(SDW) 
