јвторизаци€
ѕоиск по указател€м
Farges J. (ed.) Ч Organic conductors: fundamentals and applications
ќбсудите книгу на научном форуме Ќашли опечатку?
Ќазвание: Organic conductors: fundamentals and applicationsјвтор: Farges J. (ed.) јннотаци€: Discussing the key issues that distinguish organic conductors from more conventional conductive compounds, this comprehensive and up-to-date reference examines all aspects of organic conductors;detailing the most recent theoretical concepts and current laboratory methods of synthesis, measurement, control, and analysis. Describes the latest advances in molecular-scale engineering, including switching and memory systems, Schottky and electroluminescent diodes, field-effect transistors, and photovoltaic devices and solar cells! Focusing on basic chemical and physical properties, Organic Conductors covers the theory of low-dimensional conductors delineates how organic conductors fit in with the rest of condensed-matter physics investigates the molecular design and synthesis of organic conductors analyzes techniques to characterize organic conductors such as crystallographic measurements and electronic and molecular spectroscopies reviews the magnetic electron spin resonance and nuclear magnetic resonance properties or organic conductors explores the physical properties of organic conductors such as electronic instabilities and phase transitions considers the potential application of organic materials and polymers in electronics provides a summary of organic superconductors, including fullerenes and more!
язык: –убрика: ‘изика /‘изика твЄрдого тела /ѕриложени€ /—татус предметного указател€: √отов указатель с номерами страниц ed2k: ed2k stats √од издани€: 1994 оличество страниц: 854ƒобавлена в каталог: 21.08.2005ќперации: ѕоложить на полку |
—копировать ссылку дл€ форума | —копировать ID
ѕредметный указатель
Electron gas, system in high magnetic field 475Ч484 Electron gas, system of spinless fermions 416 457 Electron gas, system, electron-hole pair formation 14 409 579 585 587 602 626 627 Electron gas, system, electron-hole symmetry 515 Electron gas, system, electronic states 597Ч599 607Ч613 Electron gas, system, interacting 44 409 412 416 421 422 454 Electron gas, system, two dimensional 430 476Ч484 Electron spin resonance (ESR) 215 276Ч280 288Ч289 305 370 379 423 511 518 658 669Ч676 704Ч705 764Ч772 Electron spin resonance (ESR), g-tensor 276Ч278 286 287 294 373 704 767 Electron spin resonance (ESR), g-tensor, g shift 277 704 Electron spin resonance (ESR), linewidth 276Ч278 289 293 303Ч305 370 380 704 Electron spin resonance (ESR), linewidth, narrowness in organic conductors 288 Electron spin resonance (ESR), measurement 279 371 Electron spin resonance (ESR), relaxation times 276 277 284 Electron spin resonance (ESR), spin-orbit coupling 289 304 305 Elliot relation 278 Emeraldine 552 557 651 ENDOR experiments 511 671 Energy gap/activation energy 4 5 11 13 34Ч67 197 247 320Ч345 348 406Ч481 495Ч517 570Ч632 660 666 681 683 694Ч707 763Ч765 780 792 794 811 see "Organic Energy gap/activation energy, charge/spin excitation 34Ч36 40 41 52Ч55 63 64 263 373 Energy gap/activation energy, correlation gap 422 459 Energy gap/activation energy, dimerization (or bond-order) gap 13 48Ч52 63 65 434 Energy gap/activation energy, gap function 314 315 Energy gap/activation energy, gap states 575Ч578 591 592 604 Energy gap/activation energy, midgap states 49 51 511 575 609 622 670 Energy gap/activation energy, pseudogap 45 46 57 256 286 289 297 423 Enzymes 2 719 720 ESR see "Electron spin resonance" Exchange 67 271Ч296 299 301 327Ч332 416 447 451 764 765 Exchange, superexchange 273 Excimers 805 Excitations 238Ч242 255 501 506 517 518 570 579 589 592 617 628 670 Excitations, charge/spin 34Ч36 40Ч42 46 54 55 63 64 373 415 422 503 516 Excitations, collective 34 272 278 369 463 Excitations, gapless 26 27 33Ч35 49 Excitations, localized 517 520 521 587 Excitations, magnons 463 Excitations, nonlinear 671 683 Excitations, spectrum 45 Excitations, spin-charge decoupling 8 34 415Ч418 Excitations, spin-wave 373 excitons 516Ч518 579 585Ч597 621Ч633 708 792Ч795 811 813 817 Excitons, singlet 516 517 575Ч592 626 628 632 Excitons, singlet, exciton fission 518 579 580 Excitons, triplet 575Ч591 626 Excitons, triplet, exciton fusion 518 626 Excitons, triplet-triplet transition 581 Extended pair approximation (EPA) 664 Extensive property 497 External potential 333 Fermi, density of states 34 35 46 60 452 586 591 593 613 663 664 679 681 Fermi, density of states, singularity (van Hove) 60 61 586 Fermi, density of states, tails 576 598 611 Fermi, golden rule 434 Fermi, liquid 61 416 417 429 Fermi, liquid, non-Fermi liquid behavior 429 Fermi, surface 6 11 17 28Ч67 148Ч149 164 181 193 197Ч199 245 270Ч284 319 336 365Ч367 380 406Ч484 598Ч624 651 653 663 679Ч681 696 795 806 813 819 Fermi, surface, closed 59Ч61 65 197 198 Fermi, surface, nesting 12 17 34 58Ч66 197 198 366 380 428Ч481 Fermi, surface, open 30 65 197 366 Fermi, surface, pinning 607 Fermi, surface, quantized nesting theory 477 Fermi, surface, two-dimensional 17 32 58 429Ч430 439Ч446 Ferrimagnetic state 272 273 Ferrocenes 695 Ferromagnetic state 26 61 67 68 272 297 299 412 413 690 Flavins 719 720 Fluctuations 10 17 18 26Ч62 177Ч190 218 281 369 411Ч427 446 451 462Ч463 500 520 545 623 668 768 see Fluctuations, antiferromagnetic 421 422 434 447 Fluctuations, enhancement 477 Fluctuations, pretransitional 148 179Ч192 284Ч296 331 332 462Ч463 Fluctuations, quantum 27Ч68 Fluctuations, superconducting 43 52Ч53 Fluctuations, thermal 26 27 45Ч48 55 57 62 68 413 Fluctuations, three dimensional 427 430 Fluoranthene 304 Fluorenones 805 Free radicals 511 512 581 671 704 719 see Froelich mode 46 55 62 65 463 Fullerenes 19 32Ч33 67Ч68 76 96 116 134 405Ч409 522 736 Fullerenes, 9 68 134 136 409 451 452 487 736 775 Fullerenes, 451Ч452 Fullerenes, 136 Fullerenes, LB films 762 775Ч776 Fullerenes, microtubes 33 Fullerenes, preparation route 409 G-ology model 30 37 410 G-ology model, diagrammatic representation 410 414 Glass, metallic 396 Glycine 696 graphite 304 405 see Ground state, degenerate versus nondegenerate 508Ч513 520 551 575 585 622 669Ч684 Hall effect 64 366Ч368 383Ч387 436Ч440 Hall effect, measurements 383Ч386 Hall effect, quantized 64 387 476Ч481 Hall effect, quantized, difference with ordinary quantum effect 481 Hall effect, quantized, sign change 387 477 Hartree Ч Fock approximation 13 272 275 Heisenberg chain 272 275 288 301 327Ч328 331 422 Heisenberg chain, random-exchange, antiferromagnetic (REHAC) 764 765 Heterocycles 82 Hexamer 582 Hexathioorthooxates 122 125 High temperature superconductors (HTSC) 9 32 35 59Ч68 312 397 735Ч757 High temperature superconductors (HTSC), a summary 736 High temperature superconductors (HTSC), comparison with organics 17Ч18 67 68 736Ч737 High temperature superconductors (HTSC), substitutions in 746Ч748 High temperature superconductors (HTSC), substitutions in 748Ч750 High temperature superconductors (HTSC), substitutions in 744Ч746 High temperature superconductors (HTSC), substitutions in 740Ч742 High temperature superconductors (HTSC), substitutions in 742Ч744 High temperature superconductors (HTSC), substitutions in 737Ч740 High temperature superconductors (HTSC), Tl-Ba-Ca-Cu-O system (thallium salts) 735Ч757 High temperature superconductors (HTSC), toxicity aspects 737 750 High temperature superconductors (HTSC), Y-Ba-Cu-O system 17 200 735 HMTSF 365 HMTSF-TCNQ 287Ч289 365 371 380 383 387 388 HMTSF-TNAP 288 373 HMTTF-TCNQ 373 380 Holons 35 Hopping 28Ч30 55Ч66 283 288 377 390 395 434 499 599 625Ч667 680 698 797 Hopping, models 394 396 798 Hopping, models, variable range hopping (VRH) 15 303 663Ч666 762 773 Hubbard model 5 29Ч64 230 239 274Ч288 312 319 330 373 406 415 416 422 454Ч459 487 507 589 590 764 see Hubbard model, extended 29 30 274 275 285 330 Hubbard model, two-dimensional 32 60 61 64 67 Hume-Rothery rules 12 Hund rule 68 Hydrazones 805 Hydroxydinitropyridines 692 Hysteresis see "Phase transitions" Impurities see "Disorder" Instabilities 10Ч18 63 65 150 179 230 255Ч256 270 283Ч285 292 296 313 380 408 412Ч418 479 Instabilities, one-dimensional, summary 284Ч285 interactions 27Ч33 198 230 Interactions, electron-electron/spin-spin 4Ч6 10Ч17 27Ч68 90 179 198 229 245 262 269Ч304 312Ч339 365Ч390 406Ч486 503Ч517 544Ч627 656Ч683 767 see Interactions, electron-molecular vibration (e-mv, vibronic coupling) 230Ч260 369 391Ч393 454 573 587 591 597 Interactions, electron-phonon/electron-ion/spin-phonon 29Ч66 179 192 218 229 230 245Ч255 257 262 269Ч287 312Ч333 366 369 370 381 414 451Ч452 495Ч521 544Ч590 683 Interactions, electron-phonon/electron-ion/spin-phonon, phonon softening 12 44Ч52 259 365 Interactions, screened 14Ч17 29 230 406 605 650 656 Intercalation compounds 496 502 553 648 Ion-radical salts 29Ч32 43 65 76 98 190 191 230 232 305 360 762Ч769 see Ion-radical salts, anion-radical salts 80 116 327 330 334Ч336 Ion-radical salts, cation radical salts 77Ч84 90 101 116 556 Ising chain 26 27 55 412 Isomerization, cis-to-trans, trans-to-cis 778Ч783 Isostructural materials 164 Isothianaphtalene 571 604 Isotopically substituted molecules 117 279 287 391Ч393 423 427 448Ч451 Isotopically substituted molecules, deuterated molecules 193 215 216 258 289 325 380 449 Josephson junctions 446 488 776 K-chloranil 240 Kinks 26 48 200 670 Kohler's rule 366 368 387Ч389 396 Kohler's rule, deviations from 388 Kramers Ч Kronig analysis 231 236 277 460 571 Landau levels 476 481 484 Landau Ч Ginzburg theory 27 45Ч49 53 Langmuir Ч Blodgett (LB) films 109 124 263 720 760Ч776 Langmuir Ч Blodgett (LB) films, air-liquid interface 760Ч764 775 Langmuir Ч Blodgett (LB) films, amphiphilic molecules 760Ч762 Langmuir Ч Blodgett (LB) films, binary mixed 768 Langmuir Ч Blodgett (LB) films, charge-transfer complexes 766Ч769 Langmuir Ч Blodgett (LB) films, conductive films 761Ч776 Langmuir Ч Blodgett (LB) films, fullerene 762 775Ч776 Langmuir Ч Blodgett (LB) films, glycerine subphase 764 766 Langmuir Ч Blodgett (LB) films, interdigitation 763 Langmuir Ч Blodgett (LB) films, ion-radical salts 762Ч766 769 Langmuir Ч Blodgett (LB) films, long-chain fatty acid 762 Langmuir Ч Blodgett (LB) films, magnetic 306 Langmuir Ч Blodgett (LB) films, miscibility 760 762 Langmuir Ч Blodgett (LB) films, molecular design 760Ч762 776 Langmuir Ч Blodgett (LB) films, molecular orientation 764Ч767 768 Langmuir Ч Blodgett (LB) films, monolayers 760Ч766 775 Langmuir Ч Blodgett (LB) films, precursors 762 764 769 Langmuir Ч Blodgett (LB) films, stability 764 766 783 Langmuir Ч Blodgett (LB) films, substrate 760 Langmuir Ч Blodgett (LB) films, surface pressure 766 Langmuir Ч Blodgett (LB) films, ultrathin films 760 776 Lecithin/iodine complex 711 Libron model 369 Lindhard function 281 Lipids 711 Little's model 7 75 218 405 487 762 Living organisms 1 2 6 Luttinger liquid 35 42 411 416 m-Cresol/pyridine (quinoline) complexes 699 m-Phenylenecarbenes 299 Macrometal 19 Madelung energy 97 magnetic properties 182 198 214Ч215 269Ч306 327Ч332 365 370Ч372 379 380 486 704Ч706 764 see "Nuclear "Spin Magnetic properties, magnetization 270Ч273 297 Magnetic techniques 270 Magnetic techniques, Faraday balance 271 370 379 421 423 Magnetic techniques, SQUID magnetometer 271 370 Magnetoresistance 17 360Ч397 463 474 481Ч484 Magnetoresistance, magic angles 368 389Ч390 397 481Ч484 Malachite green 818 Malonic acid 696 Matthiessen's rule 370 Matthiessen's rule, deviations from 396 Mean field approximation (MFA) 43Ч67 319 333 366 409Ч412 417 Mean field approximation (MFA), beyond MFA 412Ч415 477 Mechanical properties, polymers 527 528 557 567 568 649 682 Mechanical properties, polymers, flexibility 545 563 Mechanical properties, polymers, intrinsic rigidity 540 545 558 563 Mechanical properties, polymers, tensile strength 564Ч566 Mechanical properties, polymers, Young's modulus 564Ч567 Melanin 695 705 Mermin Ч Wagner theorem 26 27 45 Merocyanines 815Ч818 Metallic ions 3 Metallic regime 270 Methyl-N-ethyl-benzimidazolium 213 214 249 Methylenedithiotetrathiafulvane (MDT-TTF) 125 Methylglyoxal/protein complexes 722 Methyltriphenylarsonium 237 Methyltriphenylphosphonium 205 213 214 252 261 Micelles 720Ч721 Microscopy 150 Microscopy, atomic force microscopy (AFM) 216Ч217 544 772 Microscopy, polarizing microscope 235Ч238 764 772 Microscopy, scanning electron microscopy (SEM) 542 544 764 772 Microscopy, scanning tunneling microscopy (STM) 216Ч217 542 544 Microscopy, scanning tunneling microscopy (STM), topography 216 Microtubes 33 Mixed valance salts 75 133 MnO 273 Molecular bonds/orbitals 405 408 496 692 Molecular bonds/orbitals, 3 28Ч32 405 487 496Ч510 524 533 548 571 647 651 669 670 694 695 781 Molecular bonds/orbitals, 496 500 502 694 Molecular bonds/orbitals, 500 507 Molecular bonds/orbitals, bond lengths 26 31 164 192Ч196 200 209 519 544 545 557 Molecular bonds/orbitals, bond lengths, alternation 13 506Ч513 544 548 628 670Ч672 Molecular bonds/orbitals, bonding/antibonding/nonbonding character 48Ч52 148 197 209 511 693 805 Molecular bonds/orbitals, charge-transfer bonds 693 702 703 725 Molecular bonds/orbitals, covalent bonds 5 31 495 496 507 513 553 561 568 601 608 621 692 Molecular bonds/orbitals, double bonds 87 584 Molecular bonds/orbitals, highest occupied (HOMO) 417 694 Molecular bonds/orbitals, hydrogen bonds 20 692Ч696 700 725 Molecular bonds/orbitals, ionic 692 693 Molecular bonds/orbitals, lowest unoccupied (LUMO) 594 Molecular bonds/orbitals, overlap 405 406 409 495 496 551 Molecular chains 76 98Ч102 149 155 162 312 319 338 360 406 417 495 497 507Ч518 564 764 see Molecular chains, alkyl chains 504 561 596 762 765 769 777 780 Molecular chains, cross-linking 504 540 565 648 Molecular chains, dimerization 11 15 49Ч51 63 76 77 99 161 164 184 200 234Ч260 276 292 317Ч342 370Ч376 408 418 422 433 439 454 507 510 544 548 552 583 593 633 666 671 763 769 Molecular chains, geometry 545Ч596 Molecular chains, helicity 558 596 723 Molecular chains, interrupted 659Ч662 669 Molecular chains, lengths 540 541 561 563 599 659 665 Molecular chains, macromolecules 7 540 700Ч703 708 719Ч720 see Molecular chains, n-merization 234 235 Molecular chains, rigid-body approximation 164 183Ч186 Molecular chains, segregated 29 76 102Ч108 159 244 259 260 274 Molecular chains, tetramerization 156 203 248 252 261 292 317Ч339 360 423 Molecular chains, trimerization 48 49 235 252 360 Molecular chains, two-chain systems 360 361 370 373 380 386 Molecular chains, two-dimensional 105 161 162 184 186 203 774 Molecular crystal (MC) Hamiltonian 31 43Ч53 Molecular design 75Ч114 Molecular design, prototype molecules 101Ч105 Molecular design, recent advances 105Ч108 Molecular ferromagnets 270 297Ч303 Molecular ferromagnets, coercive field 298 302 305 Molecular ferromagnets, demagnetization field 305 Molecular ferromagnets, design strategy 298 Molecular ferromagnets, domains 297 Molecular ferromagnets, examples 300Ч303 Molecular ferromagnets, ferrimagnetic approach 299 Molecular ferromagnets, hard, soft 298 Molecular ferromagnets, hysteresis 298 302 Molecular ferromagnets, magnetization 298Ч301 Molecular ferromagnets, organometallic derivatives 298 300 Molecular ferromagnets, topological degeneracy 299 Molecular ferromagnets, transparent films 298 Molecular field approximation 272 Molecular interactions 148 417 418 442 499 540 545 558 564 579 651 792 797 Molecular interactions, interchain 27 32 33 63 64 149 179 204 244 361 408 417 418 484 519 520 523 541 544 547 551 558 562 570 649 658 659 Molecular interactions, steric site inequivalence 249 252 Molecular interactions, three-dimensional 503 518Ч520 591 Molecular interactions, van der Waals 5 20 163 194 197 237 405 792 Molecular vibrations 232 234 240 242 255 257 369 391Ч393 451Ч452 587 591 617 Molecular vibrations, totally symmetric 47 232 255 257 261 391 Mulliken theory 235 252 692 Mutagens 716 N,N'-Dicyanoquinonediimine see "DCNQI" N-dimethylthiomorpholinium 249 260Ч261 see N-methyl-2,6-methylpyridinium 248 N-Methyl-N-ethylmorpholinium see "MEM(TCNQ)_{2}" N-Methylphenazinium-TCNQ see "NMP-TCNQ" N-Methylpyrrolidone (NMP) 566 N-Propylquinolinium 337 Naphtacene 4 Naphtalene 390 517 692 Naphtho-1,4-dioxin derivatives 108 Naphthoquinone 93 Neurohumoral transmitters 707 718Ч719 Nicotinamide 711 719 NMP-TCNQ 16 192 203 288
–еклама
 |
|
ќ проекте
|
|
ќ проекте



 compounds
compounds  -sexiphenyl
-sexiphenyl 



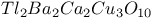

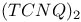



 -electron system
-electron system  -electron system
-electron system  coupling
coupling  modes
modes 