јвторизаци€
ѕоиск по указател€м
Farges J. (ed.) Ч Organic conductors: fundamentals and applications
ќбсудите книгу на научном форуме Ќашли опечатку?
Ќазвание: Organic conductors: fundamentals and applicationsјвтор: Farges J. (ed.) јннотаци€: Discussing the key issues that distinguish organic conductors from more conventional conductive compounds, this comprehensive and up-to-date reference examines all aspects of organic conductors;detailing the most recent theoretical concepts and current laboratory methods of synthesis, measurement, control, and analysis. Describes the latest advances in molecular-scale engineering, including switching and memory systems, Schottky and electroluminescent diodes, field-effect transistors, and photovoltaic devices and solar cells! Focusing on basic chemical and physical properties, Organic Conductors covers the theory of low-dimensional conductors delineates how organic conductors fit in with the rest of condensed-matter physics investigates the molecular design and synthesis of organic conductors analyzes techniques to characterize organic conductors such as crystallographic measurements and electronic and molecular spectroscopies reviews the magnetic electron spin resonance and nuclear magnetic resonance properties or organic conductors explores the physical properties of organic conductors such as electronic instabilities and phase transitions considers the potential application of organic materials and polymers in electronics provides a summary of organic superconductors, including fullerenes and more!
язык: –убрика: ‘изика /‘изика твЄрдого тела /ѕриложени€ /—татус предметного указател€: √отов указатель с номерами страниц ed2k: ed2k stats √од издани€: 1994 оличество страниц: 854ƒобавлена в каталог: 21.08.2005ќперации: ѕоложить на полку |
—копировать ссылку дл€ форума | —копировать ID
ѕредметный указатель
NMR see "Nuclear magnetic resonance" Noise generation, narrowband 463 469Ч472 Noradrenaline 719 721 Nuclear magnetic resonance (NMR) 63 66 68 177 279Ч297 420Ч434 447Ч474 544 548 705Ч706 see Nuclear magnetic resonance (NMR) in SDW state 468Ч469 Nuclear magnetic resonance (NMR), 336 423 427 452 Nuclear magnetic resonance (NMR), 279 336 551 671 672 705 Nuclear magnetic resonance (NMR), chemical shift 280 404 705 Nuclear magnetic resonance (NMR), crossover from one to three dimensional regime 283 288 Nuclear magnetic resonance (NMR), divergency 427 Nuclear magnetic resonance (NMR), dynamic nuclear polarization experiments 671 Nuclear magnetic resonance (NMR), high-resolution NMR techniques 280 705 Nuclear magnetic resonance (NMR), hyperfine interaction 280Ч283 293 420 421 Nuclear magnetic resonance (NMR), Knight shift 280 281 286 452 Nuclear magnetic resonance (NMR), Korringa relaxation 66 281 282 289 293 297 Nuclear magnetic resonance (NMR), Korringa relaxation, enhanced 429 430 Nuclear magnetic resonance (NMR), Larmor frequency 280 282 421 671 Nuclear magnetic resonance (NMR), motional narrowing 468Ч469 Nuclear magnetic resonance (NMR), Overhauser effect 672 Nuclear magnetic resonance (NMR), relaxation rate 280Ч282 335 420 423 427 429 447 Nuclear magnetic resonance (NMR), second moment 334 551 Nuclear magnetic resonance (NMR), solid-state effect 672 Nuclear magnetic resonance (NMR), strong/weak coupling 423 429 433 nucleotides 696 Octadecane 769 Octadecyldimethylsulfonium 764 Octadecylmethylethylsulfonium 764 Octadecylpyrimidium 764 Octadecyltrimethylphosphonium 764 Olefins 20 Oligomers 497 500 513 541Ч632 Optical properties, spectroscopy 229Ч267 452Ч463 539Ч544 555 559 570Ч597 658 763 768 Optical properties, spectroscopy and phase transitions 258Ч261 Optical properties, spectroscopy, absorption 231Ч258 496Ч530 559Ч563 576 577 589 631 692 702Ч704 708 764 778Ч781 792Ч817 Optical properties, spectroscopy, absorption, Benesi Ч Hildebrand equation 703Ч705 Optical properties, spectroscopy, absorption, edge 585 590 627 792 Optical properties, spectroscopy, absorption, Stokes shift 575 581 Optical properties, spectroscopy, absorption, tail 576 577 590 598 Optical properties, spectroscopy, absorption, threshold 571 574 586 600 621 Optical properties, spectroscopy, anisotropy 229Ч245 Optical properties, spectroscopy, basics 230Ч235 Optical properties, spectroscopy, birefringence 236Ч238 Optical properties, spectroscopy, conductivity 232 233 244 249 255 454 459Ч463 Optical properties, spectroscopy, conductivity, collective mode 460Ч463 Optical properties, spectroscopy, conductivity, optical gap 244Ч258 571Ч575 588Ч598 Optical properties, spectroscopy, damping rate 453 Optical properties, spectroscopy, dichroism 564 569 572 574 627 Optical properties, spectroscopy, electroluminescence (EL) 502Ч505 517 533 591 606 614Ч635 Optical properties, spectroscopy, emission 570Ч634 692 702Ч704 Optical properties, spectroscopy, fluorescence 572 574 589 628 632 633 701Ч704 792 817 Optical properties, spectroscopy, fluorescence, quenching 703 714 717 Optical properties, spectroscopy, index of refraction 231 452 Optical properties, spectroscopy, infrared 229 230 232 243 258 405 575 581 666 703 Optical properties, spectroscopy, infrared, activation of infrared nonactive modes 232Ч235 252 259Ч262 391 Optical properties, spectroscopy, infrared, far-infrared (FIR) 459Ч463 Optical properties, spectroscopy, infrared, indentations 258 Optical properties, spectroscopy, intramolecular or vibrational 229Ч242 255 261 575 587 Optical properties, spectroscopy, irradiated samples 261Ч262 Optical properties, spectroscopy, luminescence 569 571Ч575 587 Optical properties, spectroscopy, non-linear susceptibility 497 506 533 570 592 601 628 Optical properties, spectroscopy, phosphorescence 792 Optical properties, spectroscopy, photoluminescence 518 571 581 615 627 Optical properties, spectroscopy, polarizability tensor 229 235 236Ч239 Optical properties, spectroscopy, Raman light scattering 229 236 242 256Ч258 323 405 544 552 582Ч590 Optical properties, spectroscopy, Raman light scattering, resonant Raman 236 257 500 550 570 582Ч585 588 591 Optical properties, spectroscopy, reflectivity 47 229Ч236 244 245 249 252 255 259 323 333 453 459 571 Optical properties, spectroscopy, reflectivity, electroreflectance 587 589 Optical properties, spectroscopy, reflectivity, metallic 240 243 Optical properties, spectroscopy, reflectivity, plasma reflectance 243Ч245 Optical properties, spectroscopy, signatures of solitons, polarons, bipolarons 514Ч516 Optical properties, spectroscopy, solid-state spectra 571Ч573 Optical properties, spectroscopy, solution spectra 571 574 Optical properties, spectroscopy, solvatochromism 702 Optical properties, spectroscopy, spectral shift 702 703 Optical properties, spectroscopy, spectral shift, blue-shift 631 632 764 Optical properties, spectroscopy, spectral shift, red-shift 571 574 575 581 587 589 621 631 781 Optical properties, spectroscopy, thermochromism 559 560 563 702 Optical properties, spectroscopy, transitions, electronic 229 238 239 243 339 453 454 460 497 517 518 539 570Ч597 615 Optical properties, spectroscopy, transitions, electronic, oscillator strength 239 256 457Ч460 Optical properties, spectroscopy, UV-visible 236Ч243 702Ч703 Optical properties, spectroscopy, UV-visible-IR 764Ч766 769 Optical properties, spectroscopy, visible 570Ч575 585Ч591 Optical properties, spectroscopy, visible-near infrared 452Ч459 Optical techniques 235Ч236 238 Optical techniques, bolometric technique 255 Optical techniques, electron energy loss spectroscopy (EELS) 590 Optical techniques, indicatrix 229Ч239 Optical techniques, infrared absorption spectra 242 326 Optical techniques, light scattering 257 Optical techniques, microspectroscopy 238 Optical techniques, non linear optical methods 577 Optical techniques, optical detection of magnetic resonance (ODMR) 518 580 581 Optical techniques, photobleaching 579 Optical techniques, photoemission 593 604 620 621 Optical techniques, photoinduced absorption (PIA) spectroscopy 577Ч581 589Ч591 Optical techniques, photothermal deflection spectroscopy (PDS) 576 577 Optical techniques, spectrophotometers, double beam 702 Order 48 64 365 415 518 544 545 550 551 557Ч564 Order, long range 8 12 26 27 45 60Ч65 244 278 406 413 414 419 423 442 446 463 Order, parameter 6 26 27 45 46 49 55 61 62 411 413 Ordinary metals 1Ч17 230 244 271 277 281 286 365 371 396 405 447 660 776 Ordinary semiconductors (insulators) 8 233 586Ч626 660 Organic alloys 393 467 see Organic conductors 1Ч823 Organic conductors, biosynthesis 20 Organic conductors, classification 311Ч313 Organic conductors, early history 3 Organic conductors, more-than-one-dimensional 772 Organic conductors, overview 1Ч24 Organic conductors, overview, future prospects 18Ч20 Organic conductors, overview, key issues 9Ч11 Organic conductors, physical concepts 25Ч73 Organic conductors, ternary 157 244 Organic conductors, three dimensional 363 396 409 Organic conductors, two dimensional 184 186 242 245 359 361 396 406 481 Organic metal components 76 Organic metals 5Ч6 155 197 278 311 338 359Ч494 767 Organic molecules, closed/open shell 116 135 291 292 405 Organic molecules, planarity/symmetry 75 76 102 105 133 134 Organic photoconductors/photovoltaics 587 588 599 708 722 723 776 791Ч823 Organic photoconductors/photovoltaics, action spectrum 794 Organic photoconductors/photovoltaics, carrier generation 708 792Ч795 800 806Ч817 Organic photoconductors/photovoltaics, carrier generation, quantum yield 792Ч795 803 Organic photoconductors/photovoltaics, carrier transport mechanisms 797Ч798 Organic photoconductors/photovoltaics, deactivation processes 792 793 801 Organic photoconductors/photovoltaics, experimental methods 795Ч797 Organic photoconductors/photovoltaics, experimental methods, time-of-flight (TOF) techniques 795 798 Organic photoconductors/photovoltaics, experimental methods, transient techniques 795 Organic photoconductors/photovoltaics, gain 794 795 Organic photoconductors/photovoltaics, photocurrent 792Ч795 Organic photoconductors/photovoltaics, photovoltaic effect 806Ч820 Organic photoconductors/photovoltaics, sensitivity 794 795 801 Organic photoconductors/photovoltaics, threshold 588 795 Organic photoconductors/photovoltaics, transit time 794Ч799 Organic semiconductors/insulators 3Ч5 8 155 197 198 311Ч360 383 390 409 458 495Ч516 706 776 Organic semiconductors/insulators, metal-like behavior 315 336Ч337 Organic semiconductors/insulators, mixed chains materials 341Ч343 Organic semiconductors/insulators, non-metallic state 313 Organic semiconductors/insulators, segregated chains materials 315Ч341 Organic superconductors 2 6Ч9 63Ч68 76 96 99 100 108 109 115Ч138 155Ч161 179 183 204 245 255 257 263 270 289 298 312 359 392 396 405Ч494 684Ч685 762 772Ч776 Organic superconductors, BCS theory 6Ч7 44 451 Organic superconductors, BCS theory, BCS gap 60 66Ч67 257 263 333 446Ч447 Organic superconductors, BCS theory, pairing mechanism 6 41 53 60 66Ч68 446 451 486 Organic superconductors, comparison with high 17Ч18 67 68 736Ч737 Organic superconductors, isotope effect 448Ч451 Organic superconductors, Kosterlitz Ч Thouless transition 27 60 62 Organic superconductors, low magnetic field detection method 775 Organic superconductors, Meissner flux expulsion 446 Organic superconductors, penetration depth 67 Organic superconductors, precursor effect 446 Organic superconductors, s-, p-, d-waves 60 61 66Ч68 Organic superconductors, sensitivity to impurities 446 487 Organic superconductors, singlet (SS), triplet (TS) 33Ч68 283 411Ч414 Organic superconductors, specific heat anomaly 446 Organic superconductors, stabilization by repulsive interactions 412 Organic superconductors, superconducting gap, BCS gap equation 66 67 Organic superconductors, two-dimensional 156 161 179 203 359 361 369 446 484 487 488 Organic superconductors, unconventional superconductivity 67 396 487 Organic superconductors, vortices 62 488 Oxadiazoles 805 Oxalic acid 696 Oxazoles 805 p-Chloranil/p-phenylenediamine complex 693 Pariser Ч Parr Ч Pople Hamiltonian 29 Pentachloride, antimony 705 Pentamethylcyclopentadienil (or 298 Percolation mechanism 351 528 554 568 569 660 680 Periodic lattice distortions (PLD) 11 44Ч58 148 150 177Ч190 203 208 218 283 284 406 413 414 418 427 428 465 see "Phase Periodic lattice distortions (PLD), displacive modulations 181 218 Periodic lattice distortions (PLD), incommensurate 44Ч46 162 177Ч190 218 256 373 380 413 427 465 Periodic lattice distortions (PLD), intramolecular 183 247 Periodic lattice distortions (PLD), magnetic modulation 427 463 476 481 Periodic lattice distortions (PLD), quenched 46 Periodic lattice distortions (PLD), superstructures 63 161 181Ч191 203 205 256 257 291 335 Pernigraniline 503 509 Perylene 801 818 819 Perylene salts 304 408 488 Perylene-bromide complex 4 Pharmacologically active agents 707 Phase transitions 7 71 179Ч191 208 259 283Ч285 311 315 320Ч324 330Ч331 335 363Ч365 373 380 392 417 423 see Phase transitions, and broken symmetry 25Ч27 319 Phase transitions, anion (or counterion) ordering 63 179Ч203 389 442Ч444 477Ч481 Phase transitions, commensurate-incommensurate 62 149 158 Phase transitions, competition between superconducting and magnetic (dielectric) phase 362 387 409 429 446 486 Phase transitions, crystallographic study 147Ч164 177Ч190 Phase transitions, first-order 205 259 476 675 Phase transitions, generic phase diagram 417Ч420 Phase transitions, hysteresis 380 476 Phase transitions, metal-to-semiconductor/insulator 13Ч15 63 98 99 208 260 269Ч297 313 336 337 387 418Ч476 657 680 772 Phase transitions, metal-to-semiconductor/insulator, Anderson localization 378 658 661 662 Phase transitions, metal-to-semiconductor/insulator, localization under very high magnetic field 487 Phase transitions, metal-to-semiconductor/insulator, Mott Ч Hubbard localization 15 284 291 313 328 336 339 416 422 431 454 458 765 Phase transitions, metal-to-semiconductor/insulator, Wigner crystal 487 Phase transitions, metal-to-superconductor 61Ч67 235 269 274 313 387 408Ч463 Phase transitions, neutral-to-ionic transition 341Ч343 Phase transitions, neutral-to-ionic transition, color change 341 Phase transitions, order-disorder 149 161 179 180 204 261 335 Phase transitions, Peierls ( 11Ч13 16 44 61Ч66 98 148 158 180Ч208 233Ч260 283Ч296 313Ч333 339 363Ч392 413Ч418 495 503Ч510 583 585 660 671 683 767 772 Phase transitions, Peierls ( 260 336 Phase transitions, Peierls ( 204 Phase transitions, reentrance 477 481 487 Phase transitions, semiconductor-to-semiconductor 313 314 333 Phase transitions, spin-Peierls 63Ч64 148 180 188 204 205 216 259 283Ч296 327Ч342 418Ч427 Phasons 47Ч49 427 465 473 Phenothiazines 695 705 Phenyl ring 631 Phonon, anomalies 258 260 325 Phonon, softening see "Interactions" Photosynthesis 723 724 Phtalocyanine dyes 803 815Ч819 Phtalocyanines (PHTH)s 304 695 717 718 723 Physical concepts of organic conductors 25Ч73 Physical concepts of organic conductors, low-dimensional physics 26Ч55 Physical concepts of organic conductors, physics in one dimension 33Ч55 270 366 488 503 658 660 Physical concepts of organic conductors, physics in three dimensions 62Ч68 Physical concepts of organic conductors, physics in two dimensions 55Ч62 Physical concepts of organic conductors, quasi one-dimensional conductors 29Ч32 55Ч59 66 Physical concepts of organic conductors, quasi one-dimensional conductors, classical crossover 57Ч58 Physical concepts of organic conductors, quasi one-dimensional conductors, dimensionality crossover 55Ч58 Physical concepts of organic conductors, quasi one-dimensional conductors, single particle crossover 55Ч56 Physical concepts of organic conductors, quasi one-dimensional conductors, two particle crossover 56Ч57 Physical concepts of organic conductors, quasi one-dimensional conductors, two-dimensional regime 58Ч59 pinning 47 55 65 461 465Ч473 Plasma edge/frequency 230 235 244 245 368 374 453 454 457 458 461 Plasmon 244 454 Plastics, conducting 501 504 Polarizable molecules 7 15 405 715 Polarization energy 6 369 593 Polaron 51 65 254 503Ч523 544 577Ч627 656Ч684 Polaron, binding energy 513 516 519 589 623 624 Polaron, bipolaron 51 52 65 503Ч521 579Ч628 656Ч684 Poly(3-hexylthiophene)/isocaicacid LB films 776 Polyacetylene (prototype polymer) 2 10 26 258 500Ч526 529Ч628 648Ч685 Polyacetylene (prototype polymer), cis-PA 502Ч520 547 672 Polyacetylene (prototype polymer), iodine-doped 8 258 496 554 666 667 Polyacetylene (prototype polymer), isomerization cis-trans 670 Polyacetylene (prototype polymer), n-doped by alkali ions 554 666 Polyacetylene (prototype polymer), new synthesis 649 662 Polyacetylene (prototype polymer), p-doped trans PA 554Ч556 Polyacetylene (prototype polymer), stretching of 564Ч565 Polyacetylene (prototype polymer), trans-PA 508Ч520 544Ч615 658Ч684 Polyalkylthiophene 541 609 613 774 Polyaniline (PAni) 501Ч504 522 526Ч532 540Ч621 651Ч680 769 Polyaromatics 801 Polycarbenes 300 Polycarbonates 805 Polycyano compounds 95 Polydiacetylenes (PDA)s 500Ч521 546 572Ч628 Polyenes 7 497Ч521 583Ч628 670 Polyesters 805 Polyethylene (PE) 500 527 569 581 593 Polyheptadiyne 503 509 Polymeric films 502Ч504 528Ч532 539Ч613 651 652 658 676 681 Polymeric films in situ studies 651 Polymeric films, electropolymerization 769 Polymeric films, extrusion molded 570 Polymeric films, fibrillar 501 502 576 602 Polymeric films, heterogeneous 576 608 Polymeric films, homogeneous 506 570 608 622 Polymeric films, LB films 769 Polymeric films, melt processed 504 Polymeric films, membranes 529 Polymeric films, self-standing 539 Polymeric films, stretched 544 564Ч566 649 667 Polymeric films, thickness 609 610 622 Polymeric films, transparent 528 Polymers 7 8 13Ч19 26 31 32 42 49 61 65 236 256Ч263 339 344 352 405 495Ч689 Polymers in solution 521 540 541 558 569Ч572 597 615 650 651 803 Polymers in the melt 540 559 563Ч568 Polymers, blends 505 564 568Ч570 581 774 797 805 816 Polymers, catalytic properties 505 Polymers, chirality 501 505 Polymers, coherence lengths 551Ч565 Polymers, conjugated, doped (conducting) 647Ч689 Polymers, conjugated, undoped (semiconducting) 539Ч646 Polymers, conjugation 496Ч501 520 524 540Ч631 662 669 670 Polymers, conjugation, box model 499 500 Polymers, conjugation, breaking 499 521 Polymers, conjugation, lengths 497Ч500 517 520 521 540Ч631 667 Polymers, copolymers 505 540 568Ч570 632 633 Polymers, copolymers, block, diblock 505 568 Polymers, copolymers, CP-non-CP 505 569 Polymers, copolymers, donor-acceptor 719 Polymers, electropolymerization 769 Polymers, ferromagnetic polymers 298 300 306 Polymers, gel formation 569 572 Polymers, glass transition temperature 559 565 566 Polymers, homopolymers 540 Polymers, isomers 497 Polymers, liquid crystallinity 505 Polymers, macromolecules 545 658 669 Polymers, macrosalt 648 Polymers, molecular weights 540 558 560 569 Polymers, molecular wire 658 660Ч661 Polymers, new materials 501Ч503 Polymers, nonconjugated 497 500 528 Polymers, perfect 497 499 541 545 668 Polymers, persistence length 521 Polymers, piezoelectric 532 Polymers, repeat unit 496Ч521 540Ч613
–еклама
 |
|
ќ проекте
|
|
ќ проекте



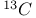
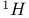
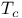 superconducting oxides
superconducting oxides  )
) 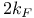 ,
, 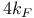 )
) 