|
|
 |
| јвторизаци€ |
|
|
 |
| ѕоиск по указател€м |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Galwey A.K. Ч Thermal Decomposition of Ionic Solids |
|
|
 |
| ѕредметный указатель |
Abnormal reactions 123Ч124
Absolute reaction rate theory 117 124Ч125 133Ч134
Absolute reaction rate theory, hydroxyhalide decompositions 375
Absolute reaction rate theory, potassium permanganate decomposition 382
Acceleratory kinetic rate equations 102Ч111
Acceleratory period 106Ч110 142
Acetate ion, decomposition 450Ч451
Acetates, metal, decompositions 448Ч451
Acid metal fluorides, decompositions 374Ч375
Activated complex 117 124Ч126
Activation energies for metal oxalate decompositions 468
Activation energies, oxyhalide decompositions 376
Activation energy 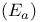 117Ч134 199 567G 117Ч134 199 567G
Activation energy, band structure, chromate decomposition 389Ч390 404
Activation energy, carbonate decomposition 345Ч346
Activation energy, nickel carboxylate decomposition 483
Activation energy, perchlorate decomposition 366
Active reaction zone 126Ч129
Additives and silver oxalate decomposition 457
Additives in lead azide decomposition 336Ч337
Additives, specific 192
Adsorbed species 47Ч48
Adsorbed species in metal promoted reactions 544
Adsorbed species, mobile 125
Adsorbed water 229 241
Alkali metals see УGroup IA metalsФ
Alkaline earth metals see УGroup IIA metalsФ
Alpha (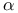 ) see also Уfractional reactionФ 30 60 570G ) see also Уfractional reactionФ 30 60 570G
Alum dehydrations 236Ч241 251
Aluminium hydride, decomposition 316 533
Aluminium hydride, irradiation 316
Aluminium hydroxides, dehydration 273Ч275
Aluminium sulfate hydrate, dehydration 235
Aluminium sulfate, decomposition 401Ч402 405
Ammine chromium(III) halides and nitrate, decompositions 504Ч506
Ammine cobalt(II) halides, nitrate and sulfate, decompositions 503
Ammine cobalt(III) azides,decompositions 500Ч502 521
Ammine cobalt(III) hydrate salts, decompositions 500
Ammine cobalt(III) iodides, decompositions 498Ч499
Ammine nickel coordination compounds, decompositions 507Ч508
Ammine palladium coordination compounds, decompositions 507
Ammine platinum coordination compounds, decompositions 507
Ammonia decomposition, iron nitride 320
Ammonium aluminium sulfate, basic, decomposition 426
Ammonium azide, thermal reactions 431
Ammonium bicarbonate, decomposition 431
Ammonium bromate, decomposition 423
Ammonium carbonate, decomposition 431
Ammonium carboxylates, decompositions 431Ч432
Ammonium chlorate, decomposition 423
Ammonium chromate, decomposition 428Ч430
Ammonium copper sulfate hexahydrate, dehydration 229
Ammonium dichromate, decomposition 429Ч430
Ammonium dichromate, decomposition, melting 430
Ammonium halates, decompositions 422Ч423
Ammonium iodate, decomposition 423
Ammonium molybdates, decompositions 432
Ammonium nitrate in ammonium salt decompositions 423 429Ч430 434Ч435
Ammonium nitrate, decomposition 424
Ammonium oxalate monohydrate, dehydration 248
Ammonium perchlorate, decomposition, catalyzed 419Ч420
Ammonium perchlorate, decomposition, high temperature 415Ч420
Ammonium perchlorate, decomposition, low temperature 415Ч420 508
Ammonium perchlorate, decomposition, mechanisms 418Ч420
Ammonium perchlorate, dislocations 416Ч417
Ammonium perchlorate, microscopy 416Ч417
Ammonium perchlorate, nucleation 416
Ammonium perchlorate, pre-irradiation 417
Ammonium perchlorate, proton transfer 417Ч419
Ammonium perchlorate, unified reaction scheme 417Ч418
Ammonium permanganate, decomposition 430Ч431
Ammonium permanganate, irradiation 431
Ammonium peroxovanadate, decomposition 428
Ammonium perrhenates, decompositions 432Ч433
Ammonium phosphate, anion condensation 425
Ammonium phosphate, decompositions 425Ч426
Ammonium phthalates, decompositions 432
Ammonium salt decompositions, sublimation 418Ч419 431 434
Ammonium salts with oxidizing anions, decompositions 545
Ammonium salts, decompositions 415Ч435
Ammonium salts, decompositions, nitrate formation 545
Ammonium salts, decompositions, proton transfer 419 423Ч425 428 434
Ammonium sulfate, decomposition 399 426
Ammonium tungstates, decompositions 432
Ammonium uranates, decompositions 433Ч434
Ammonium vanadates, decompositions 427Ч428
Anation reactions in coordination compounds 500 506 510Ч511 518 522
Anionic electron transfer, potassium permanganate 383
annealing 24 32 51 571G
Annealing and imperfection loss 558
Annealing, silver oxide 299
Aragonite-calcite transition 345Ч346
Arrhenius equation 48 67 117Ч134 567G
Arrhenius parameters 118Ч125 148Ч149 153Ч156 163Ч164 222 247 282 284
Arrhenius parameters, anomalous 223
Arrhenius parameters, decompositions of solids 529 541Ч542 551 557 559
Arrhenius parameters, normal 223 237
Arrhenius parameters, reactant area 190
Arrhenius plots 122Ч123 144Ч145 163
Atmosphere, influence on kinetic measurements 535Ч537
Auger electron spectroscopy 177Ч178
Austin Ч Rickett kinetic rate equation 95Ч96
Autocatalysis in solid decompositions 557
Autocatalytic reactions 94Ч96
Avrami Ч Erofeev equation exponents 144
Avrami Ч Erofeev rate equation see also УJMAEK equationФ 89 91 96 99 103 107Ч108 542
Azide decomposition, exciton formation 341
Azide decomposition, radiation generation 340
Azides, band structures 329Ч330
Azides, chemical and physical properties 330
Azides, cobalt(III) ammines, decompositions 500Ч502 521
Azides, conductivites 329
Azides, crystal imperfections 329Ч330
Azides, crystal structures 330
Azides, decompositions 329Ч341
Azides, dissociative evaporation 340
Azides, Group IIA metal, decompositions 331Ч333
Azides, nucleation and growth 329
Azides, optical properties 329
Azides, radiolysis 329Ч330
Band structure, chromate decompositions 389Ч390 404
Band structure, interface 127Ч129
band theory 6 17Ч22
Barium acetate, decomposition 450
Barium azide, decomposition 331Ч333 339Ч340
Barium bromate, decomposition 372
Barium carbonate, decomposition 350Ч351 358
Barium chloride hydrates, dehydration 242
Barium chlorite, decomposition 374
Barium copper oxalate, decomposition 466
Barium hydrogen oxalate hydrate, dehydration 248
Barium iodate, decomposition 372
Barium nitrite, decomposition 395
Barium oxalate dihydrate, dehydration 248
Barium oxalate, decomposition 462Ч463
Barium perchlorate, decomposition 367
Barium permanganate, decomposition 388
Barium permanganate, decomposition, irradiation 388
Barium peroxide, decomposition 298Ч299
Barium styphnate trihydrate, dehydration 252
Barium styphnate, decomposition 477
Barium sulfate, decomposition 399 405
Barium titanyl oxalate, decomposition 466
Barrier layer, product 96Ч100
Beryllium hydride, decomposition 315
Beryllium hydroxide, dehydration 273
Beryllium sulfate, decomposition 400 405
Bidentate ligands 512
Bimolecular reactions 125
Binary compounds, decompositions 313Ч325
| Binary compounds, stabilities 323
Binary compounds, sublimation 323Ч325
Binuclear coordination compounds, decompositions 517
Binuclear coordination compounds, formation 511
Block structures 306Ч307
Bond activation 129
Bond redistribution steps (Table 18.1) 126Ч127 133Ч134 199 200 530Ч532 542
Borate hydrate, dehydration 244Ч245
Borax, dehydration 244
Borchart and Daniels method of kinetic analysis 159Ч160
Bose Ч Einstein statistics 127Ч128
Boundary layer, surface 76
Bubble development 230Ч231
Burger's vector 14 24
Cadmium acetate, decomposition 450
Cadmium basic halides, decompositions 376
Cadmium carbonate, decomposition 355 358
Cadmium cyanamide, decomposition 338
Cadmium hydroxide, dehydration 276
Cadmium hydroxide, sintering and impurities 276
Cadmium hydroxide, unreacted crystals 276
Cadmium iodide, decomposition 374
Cadmium oxalate, decomposition 463Ч464
Cadmium oxide, decomposition 301
Cadmium sulfide, sublimation 44
Caesium azide, decomposition 335
Caesium bromate, decomposition 371 374
Caesium graphite, decomposition 319Ч320
Caesium orthophosphate, decomposition 397
Caesium periodate, decomposition 370
Caesium permanganate, decomposition 383 386Ч387
Cage effect 47
Calcite see Уcalcium carbonateФ
Calcium acetate, decomposition 450
Calcium azide, decomposition 333 339
Calcium carbonate, decomposition 132Ч133 346Ч349 357Ч358 360 533 547
Calcium carbonate, decomposition, Arrhenius parameters 347Ч348
Calcium carbonate, decomposition, diffusion 348
Calcium carbonate, decomposition, heat transfer 348
Calcium carbonate, decomposition, reversibility 346Ч349
Calcium carbonate, finely divided calcium oxide 346 348
Calcium carbonate, impurities 346Ч347
Calcium carbonate, nucleation and dislocations 346Ч347 360Ч361
Calcium carbonate, reaction interface 346 360
Calcium fumarate, decomposition 475 485
Calcium hydrogen phosphate, decomposition 397
Calcium hydroxide, dehydration 272Ч274
Calcium iodate, decomposition 372
Calcium maleate, decomposition 475 485
Calcium malonate, decomposition 475 485 542Ч543
Calcium nitrate, decomposition 392Ч394
Calcium nitrate, decomposition, metal oxide catalyzed 393Ч394
Calcium oxalate hydrate, dehydration 247Ч248 559
Calcium oxalate, decomposition 462Ч463 486
Calcium oxide from calcite decomposition 547
Calcium perchlorate, decomposition 368
Calcium peroxide, decomposition 298
Calcium propionate, decomposition 451
Calcium sulfate dihydrate, dehydration 232Ч234
Calcium sulfite, decomposition 402Ч403
Calcium-magnesium oxide from dolomite decomposition 350
Carbide decompositions 317Ч320
Carbonates, decompositions 345Ч361
Carbonates, Group IA metal, decomposition 351Ч352
Carbonates, Group IIA metal,decompositions 345Ч351 358Ч361
Carboxylate decomposition, reactivity patterns 482Ч486
Carboxylate decomposition, residual solid 481Ч482
Carboxylate decompositions, controlling bond rupture step 479Ч480
Carboxylate decompositions, dehydration 478
Carboxylate decompositions, electron transfer 443 457 462 480
Carboxylate decompositions, hydrolysis 478
Carboxylate decompositions, mechanisms 479Ч480 544 557
Carboxylate decompositions, secondary reactions 479
Carboxylates, ammonium, decompositions 431Ч432
Carboxylates, metal, decompositions 54Ч55 441Ч486
Carboxylates, nickel, decompositions 475Ч476
Carroll and Manche method of kinetic analysis 157
Catalysis, metal product 544 557
Catalyst preparation 282
Catalysts, mixed oxides, permanganate decomposition 389
Catalytic decomposition, sodium and potassium chlorates 370
Chemical features, decompositions of solids 528 554
Chemical foundations of solid state reactions 202
Chemisorbed species 126
Chemisorption, oxygen on oxides 291
Chlorates, Group IA metal, decompositions 370 374
Chlorates, Group IIA metal, decompositions 371
Chlorates, metal, decompositions 370Ч371 374
Chlorites, metal, decompositions 373
Chromates, decompositions 389Ч390 404
Chrome alum, dehydration 236 253
Chromium hydride, decomposition 314Ч315
Chromium hydroxide, dehydration 277Ч278
Chromium hydroxide, topotactic oxidation 277Ч278
Chromium oxides, decompositions 302Ч303
Chromium sulfate, decomposition 400
Chromium(III) ammines, decompositions 504Ч506
Chromium(III) ethylenediamine compounds, decompositions 513Ч514
Chromium(III) oxalato ammine compounds, decompositions 516
Chromium(III) tris-N-benzoyl-N-phenylhydroxylamine, decomposition 506
Citrate, lead, decomposition 476Ч477
Classification, dehydrations of solids, (Figure 7.4) 258Ч259
Classification, reactions of solids 527Ч528 533 555 558Ч560
Clay dehydrations 283Ч287
Clays, catalytic reactions 284
Cleavage 15
Cleaved crystal faces 237
Cobalt acetate, decomposition 449
Cobalt acetate, hydrolysis 449
Cobalt carbide, decomposition 318Ч319
Cobalt carbonate, decomposition 356
Cobalt fluoride, decomposition 374
Cobalt hydroxide, dehydration 280
Cobalt iodate, decomposition 373
Cobalt malonate, decomposition 469 542Ч543
Cobalt oxalate, decomposition 454Ч455 485Ч486
Cobalt oxide, decomposition 304
Cobalt oxide, reduction 304Ч305
Cobalt phosphate hydrate, dehydration 397
Cobalt sulfate binuclear compound, decomposition 517
Cobalt sulfate, decomposition 401 406
Cobalt sulfide, decomposition 322
Cobalt(II) ammines, decompositions 503
Cobalt(II) pyridine halides, decompositions 503Ч504
Cobalt(III) ammine azides, decompositions 545 555
Cobalt(III) ammines, decompositions 495Ч502 515
Cobalt(III) bis(dioximato) compounds, decompositions 517
Cobalt(III) coordination compounds, perchlorate, decompositions 513
Cobalt(III) diammine compounds, decompositions 512Ч513
Cobalt(III) diammine compounds, dehydration 513
Cobalt(III) ethylenediamine compounds, decompositions 512Ч513
Cobalt(III) hexaammine halides, nitrate and sulfate, decompositions 495Ч499
Cobalt(III) oxalato ammine compounds, decompositions 515
Cobalt-nickel mellitate, decomposition 467
Coherent intergrowth, oxides 307
Colour centre 11
Compensation effect 48 129Ч134 235 273 284Ч285 293 541Ч542 554Ч559 569G
Compensation effect and reversibility 542
Compensation effect in coordination compound decompositions 517
Complex compounds 493
Concentrations 1 568G
Concurrent reactions 163Ч164
Concurrent reactions in decomposition 547
Conditions of reactions and coordination compounds 519 521
Conductivity and silver oxalate decomposition 456
Conductor 16Ч22
Consecutive reactions 164
Constant rate thermal analysis (CRTA) 148
Contracting area or disc rate equation 93 104
Contracting cube or volume rate equation 92 98Ч99 101Ч104 108Ч110
|
|
 |
| –еклама |
 |
|
|
 |
|
ќ проекте
|
|
ќ проекте



 )
)