|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Galwey A.K. — Thermal Decomposition of Ionic Solids |
|
|
 |
| Предметный указатель |
Silver carbonate, decomposition, doping 354 357
Silver carbonate, in silver oxide 300
Silver chlorate, decomposition 368 371 374
Silver chlorite, decomposition 368 374
Silver formate, decomposition 442 485
Silver fulminate 338
Silver fumarate, decomposition 474
Silver maleate, decomposition 474
Silver malonate decomposition 474 542—543
Silver nitrite, decomposition 395
Silver oxalate, decomposition 456—457
Silver oxide, decomposition 299—300
Silver oxide, reduction 300
Silver perchlorate, decomposition 368
Silver periodate, decomposition 370
Silver permanganate, decomposition 388
Silver permanganate, decomposition, additives 388
Silver squarate, decomposition 469
Silver, catalysis by 320
Sintering 24 32 38—40 51 190 571G
Sintering, Group IIA metal oxides 273
Sites, surface 43 46
Smith — Topley effect 221 224—228 232 246 248 261
Smith — Topley effect and sublimation model 560
Smoothing of data 140
Sodium aluminium hydride 317
Sodium azide, decomposition 334 339
Sodium bicarbonate, decomposition 351—352 358
Sodium bicarbonate, subsurface nucleation 352
Sodium calcium sulfate trihydrate, dehydration 234
Sodium carbonate monohydrate, dehydration 241
Sodium carbonate-calcium carbonate reaction 356—357
Sodium chlorate, decomposition 370
Sodium chlorite, decomposition 374
Sodium hydrogen phosphates, decompositions 396
Sodium hyponitrite, decomposition 395
Sodium metaperiodate, decomposition 369
Sodium nickel phosphate hydrate, dehydration 397
Sodium perchlorate, decomposition 366—367
Sodium perchlorate, dehydration 366
Sodium permanganate, decomposition 386—387
Sodium peroxide, decomposition 297
Sodium sulfate, decomposition 399
Sodium superoxide, decomposition 297—298
Sodium thiosulfate pentahydrate, dehydration 245
Sodium tungstate dihydrate, dehydration 245
Solid decompositions, autocatalysis 557
Solid decompositions, crystal structures 555
Solid decompositions, experimental techniques 552—554
Solid decompositions, future development 552—555
Solid decompositions, isomorphic crystals 555—556
Solid solutions 12 192 283
Solids, classification 4—7
Spectroscopic studies 538 554
Spectroscopy 175—190
Squarates, metal, decompositions 467—469
Stability of coordination compounds 517—520
Stoichiometry 221
Stoichiometry, complex reactions 535
Stoichiometry, deviation from 292
Stoichiometry, simple reactions 535
Strain and decomposition rate 513 515
Strain field 126
Strontium acetate, decomposition 450
Strontium azide, decomposition 332—333 339
Strontium carbonate, decomposition 350 358
Strontium hydroxide octahydrate, dehydration 273
Strontium iodate, decomposition 372
Strontium oxalate, decomposition 462
Strontium perchlorate tetrahydrate, dehydration 368
Strontium perchlorate, decomposition 368
Structural dehydroxylations 283
Structural reorganization 225—226 258—259
Structure collapse in strain 279
Styphnate, barium, trihydrate, dehydration 252
Styphnate, lead, monohydrate, dehydration 252—253
Styphnates, barium and lead, decompositions 477—478
Styphnates, metal, hydrates, dehydrations 252—253
Sublimation 3 32 40—48 51 134
Sublimation in ammonium salt decompositions 418—419 431 434
Sublimation model for solid decompositions 559—560
Sulfate decompositions, kinetics 398—399
Sulfate decompositions, mechanisms 398—399
Sulfates, Group IIA metal, decompositions 399—400
Sulfates, metal, decompositions 398—409
Sulfates, transition-metal, decompositions 400—401
Sulfides, metal, decompositions 321—323
Sulfite, calcium, decomposition 402—403
Surface area, potassium permanganate 382
Surface area, reactant 190 246 260
| Surface chemistry of reactant 534 554
Surface controlling reactions 313 316 324
Surface mobility 24 224
Surface modification during reactions 534 554 558
Surface reactions 47 534 554
Surface reactions, binary compounds 313 324
Surface reactivity 534 554 558
Surface species, concentrations 541
Surface textures 238—239 276
Surfaces 9 15
Surfaces and nucleation 534 554
Talc, dehydration 286
Talc, product orientation 286
Tartrates, lithium potassium, hydrates, dehydrations 547
Temperature and reaction rate 117—134
Temperature measurement 64—65
Temperature, sample 65
Thallium azide, decomposition 337 339
Thallium bromate, decomposition 373
Thallium chlorate, decomposition 368 371
Thallium chlorite, decomposition 368
Thallium cyanamide 338
Thallium perchlorate, decomposition 369
Thermal decomposition of solids 3 6 44 45 51 569G
Thermal stabilities 1 542 547 555—558
Thermal stability, prospects for theory 527 553 555—560
Thermochemical measurements 538 554
Thermochemical measurements, modulated DSC 538
Thermochemistry 66—68
Thermodynamics of bromate decompositions 373
Thermodynamics of reactions 50—59
Thermogravimetry (TG) 62
Thermomagnetometry (TM) 63 192
Theta 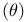 rule 130—133 569G rule 130—133 569G
Thorium formate, decomposition 446
Thorium hydride, decomposition 315
Thorium oxalate, decomposition 463
Tin oxide, decomposition 301
Tin(IV) hydroxides, dehydration 277
Titanium hydride, decomposition 315
Topochemical processes 292—293 306—308
Topochemistry 48
Topotactic preparation 49
Topotactic reactions 48—49 64 282 287
Topotactic relationships 175 189 196 199 537 554
Transition state theory 46 48 67—68 528—529 557
Transition state theory in carbonate decompositions 360
Transition state theory, perchlorate decompositions 366
Transition, displacive 34
Transition, reconstructive 34—35
Transition-metal oxides, decompositions 302—305
Transport in oxides 293 307
Trioxalatoferrate(III) potassium salt, decomposition 516
Uranium formate, decomposition 446
Uranium oxides, decompositions 305—306
Uranium oxyhydroxide, decompositions, uranyl acetate, decomposition 450—451
Uranyl formate, decomposition 446
Uranyl nitrate hydrates, dehydration 241—242
Uranyl oxalate, decomposition 459—460
Uranyl sulfate, decomposition 402 408
Valence bond 6 16—22
Vanadium pentoxide, decomposition 305
Vaporization 41—46
Vaporization, principles 40
Vaterite-calcite transition 345—346
Vermiculite, dehydration 253
Volatile species 40
Wadsley defects 307
Water bond ing 218—219
Water of crystallization 217
Water of crystallization, precursor dehydration 175 208
Water vapour atmosphere 231 271—272 275—276 284—285
Water vapour pressure 231—232
Water vapour, equilibrium pressure 271
Water, diffusion 231 242 250 258
X-ray diffraction (XRD) 64 174—175
X-ray radiolysis 179—180
X-ray topography 184—186
Yttrium formate dihydrate, dehydration 250
Zero-order rate equation 92—93 105 108—110
Zinc carbonate, decomposition 354—355
Zinc carbonate, decomposition in water vapour 354—355
Zinc formate, decomposition 445
Zinc hexacyanoferrate, decomposition 510
Zinc hydroxide, dehydration 276
Zinc hydroxide, electron beam 276
Zinc oxalate, decomposition 458—459 485
Zinc oxide, decomposition 300—301
Zinc sulfate heptahydrate, dehydration 235
Zinc sulfate, decomposition 401—2 407
Zinc sulfide, decomposition 322—323
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



 rule
rule