Авторизация
Поиск по указателям
Sidgwick N.V. — The electronic theory of valency
Обсудите книгу на научном форуме Нашли опечатку?
Название: The electronic theory of valencyАвтор: Sidgwick N.V. Аннотация: This book aims at giving a general account of the principles of valency and molecular constitlltion, founded on tI1e Rutherforcl-Bohr atom. In dev'eloping the theory of valency there are two courses open to the chemist. He may use symbols ,with no definite physical connotation to express the reacti,rity of the atoms in a molecule, and may leave it to the subseqtlent progress of science to discover what realities these symbols represent: or he may adopt the concepts of atomic physics-electrons, nuclei, and orbits-and try to explain the chemical facts in terms of these. But if he takes the latter course, as is done in this book, he must accept the physical conclusions in full, and must not assign to these entities properties which the physicists have found them not to possess: he must not use the terminology of pl1ysics unless he is prepared to recognize its laws. I have endeavoured to conform to this principle, and not to lay mysetf open to the reproach of an eminent physicist, that when chemists talk about electrons they tlSe a different lang'uage from physicists. I have been careful to avoid as far as possible the introduction of any physical llypotllcses wllich are not already sanctioned by those who are best qualified to judge of them.
Язык: Рубрика: Физика /Статус предметного указателя: Готов указатель с номерами страниц ed2k: ed2k stats Издание: 1st editionГод издания: 1929Количество страниц: 310Добавлена в каталог: 30.08.2009Операции: Положить на полку |
Скопировать ссылку для форума | Скопировать ID
Предметный указатель
Electrons, orbits of shared 98 Electrons, sharing of, Lewis's theory 56 Electrons, spinning 34 Electrons, transference of, in salts 54 Electrons, velocity of 2 Electrovalency and covalency Chapter VI 83 Electrovalency, change of, in co-ordination 111 Electrovalency, transition to covalency 92 Energy levels in atom 33 Ephraim, F. 197 200 Ethers as pure donors 137 Ethyl, sodium 260 Ewbank, E.K. 147 148 197 249 Excessive hydration 198 Fajans, K., radioactive displacement law 7 Fajans, K., theory of ionization and covalency formation 104 108 117 153 157 191 192 196 201 258 259 289 Faraday, M., diamagnetism 204 Faraday, M., laws of electrolysis 1 ferromagnetism 205 Fetkenheuer, B. 230 Fine structure of hydrogen lines 23 34 Finn, O. 221 Five-membered rings 245 Fluorides, hydration of 196 290 Fluorine 291 Fluorine, maximum covalency of 153 290 Foesterling, K. 90 Forced valency 178 Four-membered rings 243 Fowler, A. 22 23 37 Fowler, R.H. 46 98 101 French, H.S. 254 Frenkel, J. 34 Fricke, R. 190 Gallium 267 Gases, inert 257 Gases, paramagnetic 211 Geiger, H. 5 Gerlach, W. 204 208 209 Giant molecules 90 Glycocoll 245 Glyoxal 245 Goissedet, P. 169 Gold 260 262 Gold, magnetic moment of, in vapour 210 Gotts, R.A. 222 226 Goudsmit, S. 34 Grignard reagent 117 Grimm, H.G. 42 46 92 105 107 179 182 202 Grouplets, electronic 31 Groups, electronic, complete, definition of 171 Hafnium 274 Hafnium, hydrate of acetylacetonate of 169 Hafnium, theoretical importance of 44 Halides, fused, conductivity of 105 289 Halides, hydrolysis of, and covalency rule 156—158 Halides, per- 292 Halides, polymerized, structure of 244 Halides, volatility and structure of 88 Halogens 289 Hantzsch, A. 96 221 230 Harrison, P.W.B. 228 Hartree, D.R. 46 Heat of fusion and solubility 140 Heat of solution, apparent 148 Heisenberg, W. 46 Helium 257 Helium, metastable state of 257 Helium, spectrum series of ionized 22 Helium, structure of 27 Hevesy, G.V. 169 Hexafluorides, action of water on 158 Hildebrand, J.H. 139 Hoenigschmid, O. 12 Holroyd, G.W.F. 162 Holtz, J. 154 Hot spark spectra 37 Hueckel, E. 190 Hund, F. 46 214 Hydrates of acetylacetonates 169 Hydrates of inert gases 187 Hydrates of paraffins 187 Hydrates, solid 191 Hydration and size of ions 197 Hydration in solution, evidence of 190 Hydration of anions 194 Hydration of cations 192 Hydration of ions of transitional elements 216 Hydration of ions, mechanism of 189 Hydration of salts 189 Hydration of salts, and vapour pressure of solutions 197 Hydration, excessive 198 Hydration, mutual influence of ions in 196 Hydrides of boron, structure of 103 Hydrides, volatility and structure of 87 Hydrofluoric acid 291 Hydrofluoric acid, co-ordination of 72 Hydrogen 258 Hydrogen atom, Bohr's theory of Chapter II 14 Hydrogen atom, fine structure of lines of 23 24 Hydrogen atom, magnetic moment of 210 Hydrogen atom, spectral series of 21 Hydrogen atom, stationary states of 20 Hydrogen, co-ordination of 72 117 Hydrogen, covalency maximum of 153 Hydrogen, ion, hydration of 193 Hydrogen, negative ion of 64 Hydrogen, position in periodic table 63 Hydrogen, replaceability by radicals 101 Hydrolysis of halides, and the covalency rule 156—158 Hydrolysis of silicon chains 159 Hydroxyl ion, hydration of 195 Hypophosphoric acid 279 Ideal liquids 132 Increment, electronic 164 Indium 267 Inert gases 257 Inert gases, atomic numbers of 28 Inert gases, hydrates of 187 Inert gases, importance of 28 54 Inert pair of valency electrons 178 179—181 205 207 270 273 278 285 292 Ingold, C.K. 236 237 Iodine 292 293 Iodonium compounds 67 Ionization and association 137 Ionization and dielectric constant 132 Ionization and the periodic groups 106 Ionization of platinammines 111 Ionization, anti crystal structure 90—92 106 Ionization, factors determining 76 Ionization, Fajans's theory of 104 Ionization, gaseous 10 Ionized links 52 Ionized molecules, criteria of 84—86 Ions, 59 60 Ions, co-ordination of 09 Ions, complex, magnetism of 214 Ions, distribution in periodic table 104 Ions, mechanism of hydration of 189 Ions, mutual influence of, on hydration 196 Ions, simple, magnetism of 211 Ions, size of, and hydration 197 Ions, tests for 85 Iridium 297 Iron 296 Iron, carbonyl, diamagnetic 216 Iron, magnetism of 205 Isomerism a proof of covalency 86 Isotopes of lead, properties 11 Isotopes, nature of 11 Jackson, L.C. 215 Jeans, J.H. 14 15 Job, A. 169 Joergensen, S.M. 230 Jones, H.O. 220 Joos, G. 104 Kaufmann, W. 2 Kelvin, Lord 1 Kenyon, J. 228 Kipping, F.S. 159 225 Kirmreuther, H. 230 Klemm, W. 93 105 Kopp, H. 124 125 Kossel, W., spectroscopic displacement law 37 Kossel, W., theory of polar links 53—55 57 58 Langevin, P., Theory of magnetism 205 206 Langmuir, I. 18 57 62 Laporte, O. 46 Latimer, W.M. 98 Lattice, energy of ammoniates 202 Lattice, ionized crystal- 91 Le Bel, J.A. 219 Le Blanc, M. 130 Lead 273 Lead, inert pair of electrons in 181 Lead, isotopes of 11 Lembert, M.E., hydration of salts 191 194 196 199 Lewis, G.X., negative hydrogen ion 64 Lewis, G.X., octet theory 62 Lewis, G.X., references 133 159 258 Lewis, G.X., theory of non-polar link 56—59 71 83 95 Lewis, N.B. 193 Lichtenstadt, L. 225 Lindemann, F.A. 17 34 190 Links, co-ordinate, conditions of formation 116 Links, co-ordinate, definition of 60 Links, co-ordinate, dipole character of 71 122 Links, co-ordinate, Lewis's theory of 59 Links, co-ordinate, symbol for 60 Links, co-ordinate, weakness of 121 Links, covalent, Lewis's theory of 56—59 Links, electrovalent, Kossel's theory of 54 Links, semi-polar 60 71 Links, three types of 52 61 Liquids, "polar" and "non-polar" 133 Liquids, associated, conductivity of 133 Liquids, ideal 132 Liquids, miscibility of 138 Liquids, normal and abnormal 133 Lithium 259 Lithium ion, hydration of 192 Lithium, structure of 28 38 Loegstrup, M. 169 Lorentz, H.A. 4 Lowry, T.M., references 221 254 Lowry, T.M., semi-polar link 60 71 127 Lyman, T., spectral series of hydrogen 21 Magnesium 263 Magnesium, structure of atom in compounds 172 Magnetic field, intensity of, near nucleus 204 Magnetic moment of atomic rays 208 Magnetic moment of electronic orbits 205 207 Magnetic moment of metallic vapours 208 Magnetism and molecular structure 218 Magnetism and structure of carbonyl compounds 216 Magnetism and structure of nitroxyl compounds 216 Magnetism of complex ions 214 Magnetism of simple ions 211 Magnetism, Ampere's theory of 204 Magnetism, atomic and molecular Chapter XII 204 Magnetism, Curie's laws of 205 Magnetism, Langevin's theory of 205 Magnetism, Weiss's law of 212 Magneton, Bohr's 207—208 Magneton, ratio of Bohr's to Weiss's 208 Magneton, Weiss's 207 Main Smith, J.D., distribution of electrons 34 35 101 178 Mamlock, L. 236 Manganese 294 Mann, F.G. 228 248 Marckwald, W. 219 Marsden, E. 5 Maxima of covalency Chapter IX 152 Maximum covalency and the Bohr theory 167 Maximum covalency of boron 155 Maximum covalency of elements of the first short period 153 Maximum covalency of fluorine 153 Maximum covalency of hydrogen 153 Maximum covalency of nitrogen 154 Maxwell, J.C. 14 15 McLay, A.B. 46 McLennan, J.C. 46 Means, weighted 132 Meisenheimer, J. 219 220 221 225 228 229 Mendeleeff, D., periodic table 74 75 256 Mendeleeff, D., valency and the periodic groups 51 Mercurous ion 264 Mercury 263 264 Mercury, inert electrons in 179 Merton, T.R. 12 Metallic vapours, magnetic moments of 208 Metals, mutual solubility of 151 Methyl, sodium 260 Meulenhoff, J. 222 Meyer, J. 154 Meyer, S. 213 Millikan, R.A. 18 37 Mills, W.H. 66 220 221 222 225 226 Miscibility of liquids 138 Mitchell, J.E.H. 162 Mixed and pure valency groups 170 Mixed octets 173 174 276 Mixture law and association 136 Molecular association Chapter VIII 132 Molecular volume and co-ordination 124 Molecular volume and Sugden's parachor 124 Molecules, distinction from aggregates 185 Molybdenum 288 Moore, T.S. 97 250 Mordant dyes and chelation 233 Morgan, G.T. 119 222 233 234 Moseley, H.G.J. 7 8 27 Multiplets in spectra 37 Negative vapour pressure curves 140 143 Nernst, W. 132 Nestle, K.T. 230 Neville, A. 227 Nickel 298 Nickel, carbonyl, diamagnetic 216 Nickel, magnetic moment of, in vapour 210 Niessen, K.F. 99 Niobium 281 Nitrate ion, hydration of 195 Nitrate ion, polymerization of 198 Nitric oxide, paramagnetism of 211 Nitrites, volatility of 123 Nitro-compounds, volatility of 123 Nitro-group, structure of 65 Nitrobenzene a pure donor 137 Nitrogen 276 Nitrogen dioxide 279 Nitrogen, maximum covalency of 154 Nitrogen, quinquevalent, stereochemistry of 219 220 Nitrogen, tri- and quinquevalent 65 Nitrogen, trivalent, stereochemistry of 221 Nitroxyl compounds, magnetism and structure 216 Non-ionized links 52 Non-polar links 52 Normal and abnormal liquids 133 Nuclear atom and atomic number Chapter I 1
Реклама
 |
|
О проекте
|
|
О проекте



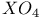 , structure of
, structure of