|
|
 |
| јвторизаци€ |
|
|
 |
| ѕоиск по указател€м |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Fowler R.H. Ч Statistical Mechanics: The Theory of Properties of Matter in Equilibrium |
|
|
 |
| ѕредметный указатель |
Landau 473
Lange and Robinson 547
Lange and Simon 218
Lange VinТs formula for susceptibility 461
Langevin 449
Langmuir 698 826 833 834;
Langmuir and Kingdon 354 364 372 375 377
LangmuirТs adsorption isotherm (q.v.) 828 Ч 831
Last multiplier 14
Lattice constants, of crystalline salts, calcuђlated 328 Ч 332
Lauritsen see УMillikan and L.Ф
Lehrer 462
Lennard-Jones 5 292 295 299 309 620
Lennard-Jones and Cook 283 308
Lennard-Jones and Dent 332 335
Lennard-Jones and Ingham 318
Lennard-Jones and Taylor 331 332
Lewis (G. N.) 207 234 312 719
Lewis (G. N.) and Randall 228 229
Lewis (G. N.) and Smith 719
Lewis (G. N.) and Von Elbe 105
Lewis (W. C. McC.) 77 280
Limiting principle 19
Lindemann 711
Lindner see УBodenstein and L.Ф
LiouvilleТs theorem 11 Ч 12
Liquids, densities of, rectilinear diameter for 844 Ч 846
Liquids, densities of, rectilinear diameter for,dielectric constant of polar 816 Ч 825
Liquids, densities of, rectilinear diameter for,pressure effects on 529 Ч 531
Liquids, densities of, rectilinear diameter for,pure, approximate partition functions for 522 Ч 525
Liquids, densities of, rectilinear diameter for,scattering of light by 765 Ч 769
Liquids, densities of, rectilinear diameter for,structure of strongly polar 451 Ч 453
Livens 437
Local order 800 Ч 801
Localized systems, partition functions for 39
LOCK see УFowler and L.Ф
London 296 847;
Long range order in a lattice 792 Ч 801
Lorentz 404 437 440 740 765
LorentzТs formula for susceptibility 441
LorentzТs formula for susceptibility, lemma 440 Ч 441
Love 127
M ller see УAngerer and M.Ф ller see УAngerer and M.Ф
MacDougall see УGiauque and M.Ф
MacGillavry 234
Madelung 314
Magnetic deviation effect 507 Ч 511
Magnetic fields, dissociative equilibrium in 475 Ч 477
Magnetic fields, transverse effects of, in metals 421 Ч 426
Magnetic phenomena, thermodynamics of 481- 483
Magnetization, of crystals, (i-h) curves for 511 Ч 516
Magnetization, of crystals, (i-h) curves for, dipole and quadripole energy of 503 Ч 504
Magnetization, of crystals, (i-h) curves for, easy directions of, in crystals 602
Magnetization, of crystals, (i-h) curves for, entropy of 469 Ч 471
Magnetization, of crystals, (i-h) curves for, magnetostriction 616 Ч 521
Magnetization, of crystals, (i-h) curves for, of ferromagnetics, ideal (i-h) curves for 478
Mahajani 503
Manneback 455
Mass action, equation of 160
Mass action, equation of, with solid phases 174 Ч 175
Massey and Mohr 310 311
Mathias, Onnes and Crommelin 844 846
Maxwell 8 308
MaxwellТs distribution law 3 58
MaxwellТs distribution law, general form of 671 Ч 672
MaxwellТs distribution law, preserved by emission and absorption of radiation 733 Ч 739
MaxwellТs distribution law, with mass motion 58 Ч 60
Mayer 329; see УBorn and M.Ф УHelmholtz УHuggins
Mayer and Helmholtz 329 334
McCrea 66 91 97 611 636 637
Mecke 99 223
Melting, change of electrical conductivity of metals on 526 Ч 627
Melting, change of electrical conductivity of metals on, elementary theory of 526 Ч 526
Mendelssohn see УSimon M.
Mensing and Pauli 455
Menzel 597 636
Metals, contact potentials of 362 Ч 364
Metals, contacts of 429 Ч 432
Metals, electrical and thermal conductivities of 404 Ч 418
Metals, electron emission of 347 Ч 358
Metals, electron specific heat of 343 Ч 344 391
Metals, electron theory of 338 Ч 436
Metals, electron theory of, elementary 338 Ч 364
Metals, electron vapour pressure of 344 Ч 346
Metals, emission of positive ions by 370 Ч 373
Metals, free electrons in, defined 378 Ч 381
Metals, insulators and semi-conductors, defined 388 Ч 390
Metals, photoelectric effect for 358 Ч 362
Metals, potential energy step at surface of 354 Ч 355
Metals, thermoelectric effects in 411 Ч 418
Metals, transverse effects in 421 Ч 425
Metals, Wiedemann Ч Franz law for 410 Ч 411
Metals, УimpurityФ 390 Ч 391
Method of Planck 579 Ч 583
Millikan 698
Millikan and Eyring 357
Millikan and Lauritsen 357
Milne 6 186 265 286 585 595 596 611 620 625 630 631 635 652 724 724 725 733 734; УFowler
Milne and Chandrasekhar 611
Milner 274
Minnaert see УPannekock and M.Ф
Mitchell (A.) 827
Mitchell (K.) 359
Mitchell (S. A.) 630
Moeller 816 822
Mohr see УMassey and M.Ф
Molecules, classified by last collision 624
Molk see УTannery and M.Ф
Momentum of radiation 732
Momentum transfer, fluctuations in 776 Ч 783
Monolayers, equation of state for 826 Ч 828
Monolayers, equation of state for, virial, use of, for 827
Morrell see УChapman (S.) T.
Morrie 359
Morse see УCondon and M.Ф
Mott 67 393 396 524
Mott and Jones 397
Mott-Smith and Daily 459
Moullin 785
Mulholland 80
Muller 550
Nernst 4 228
Nernst effect, defined 422
NernstТa heat theorem, enunciated 228 Ч 229
NernstТa heat theorem, enunciated, statistical basis of 230
Neumann and RegnaultТs law for solids 124
Noddack 742
Nordheim 338 349 350 356 496 672;
Nordheim and Kikuchi 672 677
Normal modes, for continuum 112 Ч 115
Normal modes, for continuum, EinsteinТs approximation for 118
Normal modes, for continuum, for linear lattice 132 Ч 133
Normal modes, for continuum, for square and cubic lattices, simple 133 Ч 138
Normal properties, defined 11
Nottingham 347 352
Nuclear transformations, assemblies with 655- 657
Nuclei, symmetry rules for 156
Nuclei, symmetry rules for, abundance lines for, of even mass number 657
Oermer see УDavison and G.Ф
Omstein and Burger 783
Omstein and Kramers 672
Oneager 269
Opalescence, critical 765
Oppenheimer 727
Oraetein see УUhlenbeck and O.Ф
Order, of neighbours in a lattice 800 Ч 801
Order, of neighbours in a lattice, superlattice 792 Ч 801
Order-disorder phenomena 789 Ч 809
Ordering effect of short range forces 798 Ч 800
Ornstein and van Wijk 785
| Orthohydrogen 85
Orthohydrogen- parahydrogen transformation rate 850 Ч 852
Otto see УHolbom and O.Ф
Overlap interatomic energy, in crystals 295 320
P lya 762 lya 762
P lyaТs theorem on fluctuations 762 lyaТs theorem on fluctuations 762
Pannekock and Minnaert 636
Parahvdrogen 85
Paramagnetism, of alkali metals 475
Paramagnetism, of alkali metals, of atomic ions 464 Ч 469
Paramagnetism, of alkali metals, of electrons, free degenerate 471 Ч 472
Paramagnetism, of alkali metals, of gadolinium sulphate 839 Ч 841
Paramagnetism, of alkali metals, of gases 463 Ч 464
Paramagnetism, of alkali metals, of nickel above curie point 480
Paramagnetism, of alkali metals, saturation of 467 Ч 469
Parameters, intensive, defined 52
Partial potentials, thermodynamic 194
Partington and Shilling 77 91 92 94 275 278
Partition function, EinsteinТs approximation to 118
Partition functions for localized systems 39
Partition functions, DebyeТs approximation to 119- 128
Partition functions, for adsorbed monolayers 834 Ч 835
Partition functions, for adsorbed monolayers, blochТs for ferromagnetics 496 Ч 501
Partition functions, for adsorbed monolayers, configurational, BetheТs approximation to 797 Ч 806
Partition functions, for adsorbed monolayers, configurational, of Bragg and Williams 791 Ч 797
Partition functions, for adsorbed monolayers, for atomic ions 561 Ч 562
Partition functions, for electrons near anucleus 572 Ч 574
Partition functions, for harmonic oscillators 40
Partition functions, for holomagnetization, process of 504 Ч 507
Partition functions, for mixed crystals 176 Ч 182
Partition functions, for potential energy of a gas 237 Ч 240
Partition functions, for radiation 115 Ч 116
Partition functions, for rotations, rigid, asymptotic formulae for 81 Ч 82
Partition functions, for vibrations of diatomic gases 89
Partition functions, HeisenbergТs, for ferromagnetics 485 Ч 491
Partition functions, long and short range order partition functions, for adsorbed monolayers, in terms of 801- 805
Partition functions, rigid 41 79
PARTS see УEucken and P.Ф
Pauli 28 456 471 727 753;
Pauling 222 322 810
PauliТs exclusion principle 28 293
Payne 594 597
Peierli 424 809 834
Peltier effect, defined 412
Peltier effect, defined, statistical theory of 413 Ч 418
Pelzer and Wigner 847
Penney 99; see УKronig and P.Ф
Perfect gases, equation of state of 77
Perfect gases, equation of state of, specific heats of (q.v.), diatomic 79 Ч 94
Perfect gases, equation of state of, specific heats of (q.v.), monatomic 78
Perfect gases, equation of state of, specific heats of (q.v.), polyatomic 94 Ч 106
Periodic field, electron states in (q.v.) 379 Ч 388
Permeability 438; see УsusceptibilityФ
Perrin 765 768 769 772
Persistence of velocities, effect of 704
Phase integrals of gibbs 65
Photochemical effects 741 Ч 742
Photoelectric effect, for fixed atoms 724 Ч 726
Photoelectric effect, for fixed atoms,for metals 358 Ч 362
Photoelectric effect, for fixed atoms,for solids 740 Ч 741
Photon theory of radiation 117 Ч 118
Plaakett 595 632
Planck 1 77 192 200 201 204 257 280 284 572 734
PlanckТs, characteristic function 192
PlanckТs, characteristic function, law for temperature radiation 112 116
PlanckТs, characteristic function, oscillator see УHarmonic oscillatorФ
Plyler and Barker 99
Poincar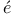 200 200
Poisson Ч Boltzmann equation, established 263
Poisson Ч Boltzmann equation, established, solved for strong electrolytes 549 Ч 552
Polanyi see УEyring and P.Ф
Polar liquids, dielectric properties of 823 -825
Polarizing force in matter 439
Positive electrons, assemblies with 653 Ч 654
Positive ions, emission of, by metals 370 Ч 373
Potential energy constants, for crystals, cubic 319
Potential energy constants, for crystals, cubic, for imperfect gases 306
Potential energy constants, for crystals, cubic, for imperfect gases, from viscosity 308 Ч 312
Potential energy, of crystalline salts 312 Ч 319
Potential energy, of crystalline salts, partition function for, of gases 237 Ч 240
Powell 503 510 516
Pressure 75
Pressure,of radiation 116
Purcell see УVan Vleck H.
Pyroelectric effect 148 Ч 149
Radiation, entropy of 116 191
Radiation, entropy of, as photons 117 Ч 118
Radiation, entropy of, momentum of emitted 732
Radiation, entropy of, partition function for temperature 115 Ч 116
Radiation, entropy of, PlanckТs law for temperature 112 116
Radiation, entropy of, pressure of 116
Radiation, entropy of, Stefan Ч Boltzmann law for 116
Radiative processes, emission and absorption, by fixed atoms 720 Ч 724
Radiative processes, emission and absorption, by free atoms 733 Ч 739
Radiative processes, emission and absorption, by solids 739 Ч 740
Radiative processes, emission and absorption,free-free transitions 726 Ч 727
Radiative processes, emission and absorption,general nature of 720 727
Radiative processes, emission and absorption,photoelectric effect for fixed atoms 724 Ч 726
Raftetti 223
Randall 560; see УLewis (G. N.) and R.Ф
Rankine 294
RaoultТs law for ideal solutions 527
Rayleigh 289 767
Reaction isobar, isotopic effects on 226 Ч 227
Reaction isobar, isotopic effects on, statistical theory of 225 Ч 228
Reaction isobar, isotopic effects on, thermodynamic theory of 225
Reaction isochore 160
Reaction rates for hydrogen and deuterium 850 Ч 852
Reactions, chemical, ArrheniusТ equation for 702
Reactions, chemical, ArrheniusТ equation for, bimolecular, EyringТs theory of 847 Ч 852
Reactions, chemical, ArrheniusТ equation for, bimolecular, simple theory of 703 Ч 706
Reactions, chemical, ArrheniusТ equation for, general nature of, in gases 700 Ч 702
Reactions, chemical, ArrheniusТ equation for, order of 701
Reactions, chemical, ArrheniusТ equation for, reverse reactions, effect of 706 Ч 707
Reactions, chemical, ArrheniusТ equation for, unimolecular 710 Ч 716
Reactions, photochemical 741 Ч 742
Rectification, general discussion of 435 Ч 436
Rectilinear diameter for liquid densities 844 - 846
Reimann 346 352 364
Relativistic energies, assemblies of electrons with 648 Ч 650
Rice see УHerzfeld and R
Richardson 345 346 357 366 370 417
Richardson Ч Einstein Ч de Haas effect 484
Rideal see УBlackett H.
Riemann 314
Righi Ч Leduc effect, defined 423
Ritter see УCrenshaw and R.Ф
Robinson see УLange and R.Ф
Rochelle salt, properties of 823
Rodebush see УHovorka and R.Ф
Roehr see УDu Bridge and R.Ф
Rosseland 585 638;
RosselandТs theorem on stellar interiors (q.v.) 638 Ч 639
Rotational freezing 824
Rotational specific heat 81 Ч 82
Rotations, rigid, classical 62 Ч 63
Rotations, rigid, classical,of molecules in solids 810 Ч 815
Rotations, rigid, classical,partition functions for 41 79
Rotations, rigid, classical,weights for 21
Rowland 779
Ruhemann see УSimon M.
Rule of equal areas 795
Rupp 354
Russell 594 615
Saha 594 595
Salow and Steiner 106
Sandved see УGronwall La
Scatchard 535 546 560
Scattering of light by liquids and gases 765- 769
Schlesinger 712
|
|
 |
| –еклама |
 |
|
|
 |
|
ќ проекте
|
|
ќ проекте



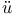 ller
ller 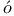 lya
lya