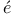|
|
 |
| јвторизаци€ |
|
|
 |
| ѕоиск по указател€м |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Fowler R.H. Ч Statistical Mechanics: The Theory of Properties of Matter in Equilibrium |
|
|
 |
| ѕредметный указатель |
 -phases, Hume Ч RotheryТs rule for 382 -phases, Hume Ч RotheryТs rule for 382
Absorption spectra, stellar 593 Ч 619
Abundance lines for nuclei of even mass number 637
Accessible states, defined 9
Accessible states, defined, enumerated 23 29
Activated complexes, number of, for bimolecular reactions 849 Ч 850
Adams 549 560
Adiabatic processes 74
Adsorbed films on surfaces 825 Ч 838
Adsorption isotherms, atomic, from diatomic gases 830 Ч 831
Adsorption isotherms, atomic, from diatomic gases, critical 833 Ч 838
Adsorption isotherms, atomic, from diatomic gases, of competing molecules 831 Ч 832
Adsorption isotherms, from diatomic gases, generalizations 832 Ч 833
Adsorption isotherms, from diatomic gases, LangmuirТs 828 Ч 831
Adsorption isotherms, from diatomic gases, molecular 828 Ч 830
Akulov 516
Anderson 364
Angerer and M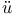 ller 334 ller 334
Antisymmetrical states 27 42
Appell 314
Appleyard see УSkinner and A.Ф
Arrhenius 293
ArrheniusТ equation for reaction rates 702
Assemblies, defined 8
Assemblies, evaporating 168 Ч 172
Assemblies, general, of crystals and vapours 173 Ч 174
Assemblies, of classical systems 56 Ч 66
Assemblies, of dissociating gases 157 Ч 168
Assemblies, of electrons and positive ions 574 Ч 583
Assemblies, of electrons with relativistic energies 648- 650
Assemblies, of harmonic oscillators 30 Ч 35
Assemblies, with nuclear transformations 655 Ч 657
Assemblies, with positive electrons 653 Ч 654
Atmospheres, free paths in upper 625 Ч 626
Atmospheres, ionized, equilibrium of 585 Ч 593
Atmospheres, of brownian particles 770
Atmospheres, planetarv, escape of molecules from 620- 630
Atomic ions, in crystals (q.v.),paramagnetism of (q.v.) 464 Ч 469 840
Atomic ions, in crystals (q.v.),partition functions for 561 Ч 562
Atomic ions, in crystals (q.v.),structure of, described 562 Ч 571
Atomic ions, in crystals (q.v.),weights of states of 569
Atomic ions, overlap and van der Waals energies of, in crystals (q.v.) 320 Ч 328
Backer and Goudsmit 601
Barker 455; see УPlyler and B.Ф
Barnes and Silverman 775 785
Barnett 484
Bartholome see УClusius and B.Ф
Becker 516 689 724
Benedicks 495
Bernal and Fowler 452 540
Bernal and Tamm 522
Berthelot (d.), equation of state of 276
Bethe 6 397 789 797;
Bid well 416
Bimolecular reactions, activated complex for 848
Bimolecular reactions, activated complex for, in gases 703 Ч 706
Bimolecular reactions, activated complex for, quantum theory of 847 Ч 852
Binary mixtures, of imperfect gases 281 Ч 283
Binary mixtures, of imperfect gases, virial coefficient (second) for 306 Ч 308
Birkhoff 712
Birtwistle 280
Bitter 513
Bjerrum 536 541 546 552
Blackett, Henry and Rideal 93
Blackman 118 120 137
Bliih and Stark 335
Bloch 338 496 502
Bloch and Gentile 503
Bodenstein and Lindner 707
Bohr 19 196 404 472 690
Boltzmann 15 189 284 666
BoltzmannТs constant 189
BoltzmannТs constant, distribution law 63
BoltzmannТs constant, equation 669
BoltzmannТs constant, H-theorem 666 Ч 669
BoltzmannТs constant, h-theorem, general form of 670 Ч 671
BoltzmannТs constant, h-theorem, quantum form of 675 Ч 677
BoltzmannТs constant, hypothesis 200 Ч 201 205
BOM 19 74 118 119 127 141 148 187 313 331
Bom and Mayer 295 319 328 329
Bonh ffer and Harteck 86 ffer and Harteck 86
Born and Brody 314
Born and Fock 74
Born and Goppert-Mayer 118 119
Bose 43 467
Bothe 672
Boundary density in imperfect gases 291
Br nsted 559 560 nsted 559 560
Bragg and Chapman 332
Bragg and Williams 6 789 808
Bridgman 418
Brillouin 338 382 468
Brody see УBom and B.Ф
Bronetein 387
Brown 223
Brownian movements 769 Ч 775
Brownian movements, distribution laws, general, for 785 Ч 788
Brownian movements, random displacements of any type 774 Ч 775
Brownian movements, rotations of mirror in a gas 779 -783
Bryan 194
Buckingham 292
Buisson see УFabry and B.Ф
Bums see УCurtis and B.Ф
Burger see УOmstein and B.Ф
Burgers 19
Burkill 198
Campbell 778
CampbellТs theorem on fluctuations 778 Ч 779
Cardwell 359
Cario and Franck 684
Cario, 684 see УFranck and C.Ф
Chandrasekhar 584 636 652;
Chapman (A. T.) see УJohnston (H. L.) and C.Ф
Chapman (S) 309 669; УTopping
Chapman (S.), and Milne 627
Chapman (S.), Topping and Morrell 332
Characteristic function, for assemblies of electrons and positive ions 574 Ч 583
Characteristic function, for assemblies of electrons and positive ions, for characteristic function, for assemblies of electrons and positive ions, Imperfect gases 257 Ч 259 283
Characteristic function, for assemblies of electrons and positive ions, method of excluded volumes 574 Ч 579
Characteristic function, for assemblies of electrons and positive ions, PlanckТs, defined 192
Chemical constant, defined 209
Chemiluminescence 741 Ч 742
Christiansen and Kramers 718
Chromosphere, calcium 630 Ч 637
Chromosphere, calcium, fully supported by radiation 632 -635
Clark see УKeesom and C.Ф
Clark and Keesom 842
Classical rotations 62 Ч 63
Classical statistics 43
Classical statistics, approximated to 55
Classical systems, assemblies of 56 Ч 66
Clausius 286
Clayton and Giauque 234
Clusius 218 320
Clusius and Bartholom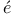 87 87
Cockcroft 838
Colby 89 495
Coleman and Egert on 218
Collisions, classical, number of given type in gases 663 Ч 665
Collisions, classical, preserving equilibrium 659
Collisions, first and second kind of 677 Ч 682
Collisions, general 2- and 3-body dissociating 691 Ч 695
Collisions, inelastic and superelastic, for electrons and atoms 677 682
Collisions, inelastic and superelastic, for heavy particles 682 Ч 684
Collisions, inelastic, applications of 684 Ч 685
Collisions, ionization by electron 685 Ч 690
Collisions, of gas molecules with surfaces 697 Ч 699
Collisions, quantum reformulation of gas 672 Ч 674
Collisions, target areas for ionization 690 Ч 691
Collisions, transferable energy in 707 Ч 710
| Complexions, defined 9
Complexions, enumerated 23
Complexions, enumerated for assemblies of complex systems 153 Ч 157
Complexions, total number of, evaluated 37
Complexions, total number of, for classical systems 47
Compressibilities, of crystalline salts calculated 328 Ч 332
Compressibilities, of crystalline salts calculated, of isotropic solids 140
Compton (A. H.) 730
Compton (K. T.) 451
Compton (K. T.) and Langmuir 346
Compton effect 730- 732
Condensation of cadmium on copper 837 Ч 838
Condon and Morse 9
Configurational partition function, BetheТs approximation 797 Ч 806
Configurational partition function, BetheТs approximation, of Bragg and Williams 791 Ч 797
Configurational partition function, BetheТs approximation, specific heat 805 Ч 809
Constable 377
Contact potential, of metals 362 Ч 364
Contact potential, of metals, of semi-conductors 401 Ч 403
Contact transformations 15
Contacts, metal 429 Ч 432
Contacts, metal semi-conductor, of high trans-parency 433 Ч 434
Contacts, metal semi-conductor, of low transparency 435
Contacts, metal, metal semi-conductor, current-voltage relaђtion for 432 Ч 433
Contacts, strongly rectifying 429 Ч 436
Continuity of path, hypothesis of 8
Continuum, normal modes for 1x2 Ч 115
Cook see УHass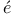 and C.Ф УLennard-Jones and C.Ф УLennard-Jones
Cooling, by adiabatic demagnetization 470- 471 841
Cooperative phenomena 789 Ч 838
Correspondence principle 19
Courant 54 114
Courant and Hilbert 550
Cox 217
Crenshaw and Ritter 814
Critical point, for imperfect gases 278 Ч 280
Crommelin see УMathias O.
Crystalline salts, properties calculated 328 Ч 332
Crystals, cubic, potential energy constants for 319
Crystals, easy directions of magnetization for 502
Crystals, entropy of 191 Ч 192
Crystals, external reactions of 138
Crystals, ferromagnetic, magnetization curves for 511 Ч 516
Crystals, general, equations of state for 141 Ч 150
Crystals, general, equations of state for, free energy of 147
Crystals, general, equations of state for, homogeneous displacements in 143
Crystals, mixed 176 Ч 182
Crystals, order of neighbours in 800 Ч 801
Crystals, overlap and van der waals energies for atomic ions in {q.v.) 320 Ч 328
Crystals, partition functions for 118 Ч 150
Crystals, potential energy per cell for (q.v.) 312 Ч 319
Crystals, strongly anisotropic, properties of 149 Ч 150
Crystals, surface forces outside 335 Ч 337
Crystals, vapour pressure of 172 Ч 173
Crystals, zero-point energy of 123
Curie point, defined 478
Curie point, defined, phenomena of 491 Ч 496
Curtis and Bums 632
Cuthbertson (C. and M.) 305
Czemy 223
Czerlineky see УGans and C.Ф
Daily see УMott-Smith and D.Ф
Dalton 64
DaltonТs distribution law 64
Darwin 1 437 444 447 473 677
Darwin and Fowler 743
Davidson 632
Davison and Germer 354
de Bruyne 356
de Haas and Gorter 844
de Haas, Wiersma and Kramers 470 839
Debye 112 118 299 437 448 450 453 460 469 730 839
Debye and H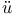 ckel 269 541 ckel 269 541
Degeneracy, relativist ic 651 Ч 652
Degenerate assemblies of electrons 71 Ч 73
Degenerate matter, equation of state of 650- 652
Degenerate systems 45
Degenerate systems, distribution laws for 45 Ч 46
Demagnetization, cooling by adiabatic 470- 471 838
Dennison 83 96
Density, great, of stellar material 647 Ч 648
Dent see УLennard-Jones and D.Ф
Derived from specific heats 197 Ч 200
Detailed balancing 659 Ч 660
Detailed balancing, in general collisions 696 Ч 697
Detailed balancing, requirements of, in gas reactions 716 Ч 719
Diamagnetism, absence of, for classical free electrons 472
Diamagnetism, absence of, for classical free electrons, of electron gas (quantum theory) 473 Ч 475
Dickinson see УTolman Y.
Dieke 84
Dielectric constant, classical theory of, for gases 447 Ч 451
Dielectric constant, for librating -rotating dipoles 817 Ч 822
Dielectric constant, for rigid rotators 455 Ч 456
Dielectric constant, for symmetrical top-like molecules 467 Ч 458
Dielectric constant, general theory of, for gas of complex molecules 458 Ч 459
Dielectric constant, isotropic property of 456 Ч 457 460
Dielectric constant, of solids and liquids with polar molecules 816 Ч 825
Dielectric constant, thermodynamic theory of 453 Ч 454
DietericiТs equation of state 276 Ч 277
Diffusion, of brownian particles 770 Ч 771
Dipole and quadripole energy of magnetization 503 Ч 504
Dipole moments of molecules 451
Dirac 9 19 25 42 696 697 721 727
Dissociation, by collisions of heavy particles 691 Ч 695
Dissociation, by collisions of heavy particles, fluctuations in 758 Ч 760
Dissociative equilibrium, in external fields 185 Ч 186
Dissociative equilibrium, in external fields, in imperfect gases 255 Ч 260
Dissociative equilibrium, in external fields, in magnetic fields 475 Ч 477
Dissociative equilibrium, in external fields, in perfect gases 157 Ч 168
Distribution laws, BoltzmannТs 63
Distribution laws, BoltzmannТs, for free electrons 64 Ч 65
Distribution laws, DaltonТs 64
Distribution laws, fluctuations in 751 Ч 755
Distribution laws, for degenerate systems 45 Ч 46
Distribution laws, for elections in overlapping bands 394Ч396
Distribution laws, for electrons, free, in metals 340 Ч 343
Distribution laws, for electrons, free, in semi-conductors 397- 401
Distribution laws, for harmonic oscillators 33 Ч 35
Distribution laws, for quantum statistics 44 Ч 45
Distribution laws, in external fields 67 Ч 69
Distribution laws, MaxwellТs (q.v.) 3 58
Distribution laws, MaxwellТs, with mass motion 58 Ч 60
Distribution laws, molecular, for imperfect gases 253 Ч 255
Donat 684
Drude 338
Du Bridge 353 361
Du Bridge and Roehr 361
Dulong and PetitТs law for solids 124
Dushman 345 352
dТOr see УEucken and dТO.Ф
Eddington 6 576 686 591 641 646 652 722
Egerton, 218 see УColeman and E.Ф
Ehrenfeat and Trkal 89 151 192 205
Ehrenfest 19 196 200
Einstein 43 118 207 720 728 753 766 769
Einstein Ч Bose statistics 43
EinsteinТs law of photochemical equivalence 742
Electrical conduction in metals, change of, on melting 526 Ч 527
Electrical conduction in metals, change of, on melting, formal theory of 404 Ч 418
Electrical conduction in metals, change of, on melting, in semi-conductors 418 Ч 421
Electrolytes, strong see Уstrong electrolytesФ
Electromagnetic theory of susceptibilities (q.v.) 437 Ч 447
Electrons, assemblies of, with relativistic energies 648 Ч 650
Electrons, assemblies of, with relativistic energies, atmospheres of 364 Ч 378
Electrons, assemblies of, with relativistic energies, degenerate assemblies of 71 Ч 73
Electrons, assemblies of, with relativistic energies, distribution laws for, in overlapping bands 394 Ч 396
Electrons, assemblies of, with relativistic energies, distribution laws for, in semi-conductors 397 Ч 401
Electrons, assemblies of, with relativistic energies, emission of, by cold metals 356 Ч 357
Electrons, assemblies of, with relativistic energies, emission of, cooling effect of 357 Ч 358
Electrons, assemblies of, with relativistic energies, emission of, the Schottky effect 355 Ч 356
|
|
 |
| –еклама |
 |
|
|
 |
|
ќ проекте
|
|
ќ проекте



 -phases, Hume Ч RotheryТs rule for
-phases, Hume Ч RotheryТs rule for  ller
ller  ffer and Harteck
ffer and Harteck