|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Wong S.S. — Chemistry of Protein Conjugation and Cross-Linking |
|
|
 |
| Предметный указатель |
S-Acetylmercaptosuccinic anhydride 19 20 251 252 259
S-Carboxymethylation 23 34
S-Sulfonate 273
S-Sulfonylcysteine 39
s-Triazines 115—117 122 307
SADP(N-Succinimidyl-(4-azidophenyl)1,3'-dithiopropionate) 167
Salicylate azides 169 see
SAND (Sulfosuccinimidyl-2-(m-azido-o-nitroben-zamido)-ethyl-1,3'-dithiopropionate) 168 238
Sandwich assays see Immunoenzymometric assays
SANPAH(N-Succinimidyl-6-(4'-azido-2'-nitrophenylamino)hexanoate) 165 235—236 239
Saporin 269
SASD (Sulfosuccinimidyl-2-(p-azidosalicylamino)ethyl-1,3'-dithiopropio-nate) 168
Schiff bases, alkylation and 60
Schiff bases, amino groups and 35
Schiff bases, carbohydrate activation and 27
Schiff bases, dialdehydes and 101
Schiff bases, heterobifunctional reagents and 182
Schiff bases, homobifunctional reagents and 133
Schiff bases, immunoconjugates and 253—254
Schiff bases, immunotoxins and 279
Schiff bases, solid matrices and 300 312
Schiff bases, zero-length cross-linkers and 203
SDS-polyacrylamide gel electrophoresis (SDS-PAGE) 213 235—239
Serine 8 12—14 17 23 see
Serine proteases 14 16 45
Serine reagents 44—45
Sialic acid 8 9
Silica 295
Site-directed mutagenesis 1
SLAB (N-Succinimidyl 4-(N-idoacetyl)amino)benzoate) 152
Small molecule cross-linking 321
SMB (N-Succinimidyl m-maleimidobenzoate) 150 277
SMBT see S-4-Succinimidyl oxycarbonyl- -methyl benzyl thiosulfate -methyl benzyl thiosulfate
SMCC see N-Succinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate
SMH see N-Succinimidyl 6-maleimidylhexanoate
SMPB see N-Succinimidyl 4-(p-maleimidophenyl)butyrate
SMPT see 4-Succinimidyloxycarbonyl- -methyl- -methyl- -(2-pyridyldithio)toluene -(2-pyridyldithio)toluene
SNAP(N-Succinimidyl-3-((2-nitro-4-azidophenyl)-2-aminoethyldithio)propionate) 168
Sodium borohydride 27 35
Sodium cyanoborohydride 27 35
Sodium dithionite 212
Solid matrices 295—313
Solid matrices, carbohydrate chain cross-linking in 312
Solid matrices, functionalities of 295—296
Solid matrices, protein immobilization by 296—312
Soluble proteins 221—228
Soluble proteins, conformational analysis and 227—228
Soluble proteins, inter-residue distance determinations and 221—223
Soluble proteins, nearest neighbor analysis and 223—227
Soluble proteins, stability of 228
Sorbitol polyglycidyl ether 122
SPDP see N-Succinimidyl 3-(2-pyridyldithio)propionate
Spin labeled bis-(N-hydroxysuccinimide) 82 83 233
Spin labels 67 68
Spin probes 245
Stability 215—217
Stability of membrane proteins 240
Stability of soluble proteins 228
Steric hindrance 14 40
Streptavidin 128—130
Structural analysis of membrane proteins 237
Substance P receptors 237
Succinate bis-(N-hydroxysuccinimide ester) 82
Succinialdehyde 93 101
Succinic anhydrides 17 296
Succinimidyl 3-(2-pyridyldithio)propionate 215
Succinimidyl 4-(p-maleidophenyl)butyrate 312
Succinimidyl 6-(N-maleimido)-n-hexanoate 215
Succinimidyl 6-maleimidocaproate 310 312
Succinimidyl acetylthioacetate 20 22
Succinimidyl acetylthiopropionate 20 22
Succinimidyl derivatives 56 see
Succinimidyl derivatives, acylation and 53
Succinimidyl derivatives, thiolation with 20 21 22
Succinyl chloride 296
Sulfates 296 see
Sulfhydryl group reagents 30—33 234
Sulfhydryl group reagents, heterobifunctional 147—162 182
Sulfhydryl group reagents, homobifunctional, alkylating agents and 111—119 121—122
Sulfhydryl group reagents, homobifunctional, disulfide reagents and 104 106—107 120
Sulfhydryl group reagents, homobifunctional, mercurial reagents and 104—106 120
Sulfhydryl group reagents, homobifunctional, nis-maleimides and 104 107—111 120—121
Sulfhydryl groups 8 10 63 215 see
Sulfhydryl groups, acylation and 37 53
Sulfhydryl groups, amine conversion to 17—20
Sulfhydryl groups, amino groups compared with 34
Sulfhydryl groups, hydroxyl groups converted to 23—25 26
Sulfhydryl groups, pH effect on 12
Sulfhydryl groups, photoactivatable reagent anchoring by 173—176 185
Sulfo-MBS (m-Maleimidobenzoyl-sulfosuccinimide ester) 277
Sulfo-SADP(Sulfosuccinimidyl-3-((4-azidophenyl)dithio)propionate) 166
Sulfo-SANPAH (N-SuIfosuccinimidyl-6-(4'-azido-2'-nitrophenylamino)hexanoate 166
Sulfo-SIAB (N-Sulfosuccinimidyl 4-(N-idoacetyl)amino)benzoate) 153
Sulfo-SMCC see N-Sulfouccinimidyl A-(N-maleimidomethyl)cyclohexane-1-carboxylate
Sulfo-SMPB (N-Sulfosuccinimidyl 4-(p-maleimidophenyl)butyrate) 149 277
Sulfonates 296
Sulfone bond cleavage 63 213
Sulfonic acids 53 see
Sulfonyl azides 128 see
Sulfonyl chlorides 38 56 100 306 see
Sulfonyl halides 31 39 90 100
Sulfosuccinimidyl 56
Sulfosuccinimidyl-2-(m-azido-o-nitrobenzamido)-ethyl-1,3'-dithiopropionate (SAND) 168 238
Sulfosuccinimidyl-2-(p-azidosalicylamido)-1,3'-dithiopropionate (SASD) 235
| Sulfosuccinimidyl-2-(p-azidosalicylamino)ethyl-1,3'-dithiopropionate (SASD) 168
Sulfosuccinimidyl-3-((4-azidophenyl)dithio)propionate (Sulfo-SADP) 166
Sulfur mustards 121 123
Tanryldi(methyl-2-aminoacetimidate (TDAA) 80
Tartryl di( -alanylazide) (TDAA) 91 -alanylazide) (TDAA) 91
Tartryl di(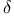 -aminovalerylazide (TDVA) 91 -aminovalerylazide (TDVA) 91
Tartryl di( -aminobutyrylazide (TDBA) 91 -aminobutyrylazide (TDBA) 91
Tartryl di(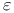 -aminocaproylazide (TDCA) 91 -aminocaproylazide (TDCA) 91
Tartryl di(glycylazide) (TDGA) 91
Tartryl diazide (TDA) 91
Tartrylbisglycinazide 69
Tartryldi(methyl-3-aminopropionimidate 80
TCEA (Tri(2-chloroethyl)amine) 115
TDA (Tartryl diazide) 91
TDAA (Tanryl di( -alanylazide)) 91 -alanylazide)) 91
TDAA (Tartryldi(methyl-2-aminoacetimidate)) 80
TDBA (Tartryl di( -aminobutyrylazide)) 91 -aminobutyrylazide)) 91
TDCA (Tartryl di(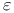 -aminocaproylazide)) 91 -aminocaproylazide)) 91
TDGA (Tartryl di(glycylazide)) 91
TDVA (Tartryl di(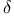 -aminovalerylazide)) 91 -aminovalerylazide)) 91
Tetranitromethane 25 126 128 202
TFA (Trifluoroacetic acid) 20
TFMS (Trifluoromethanesulfonic acid) 20
Thioester bond cleavage 63 66
Thioether 8 10
Thioether, alkylation and 53
Thioether, carboxylation and 23
Thioether, immunotoxin conjugation via 275—277
Thiol carbonate bonds 307
Thiol group reagents, heterobifunctional 310—312
Thiol group reagents, immunoconjugates and 251 254—258 260
Thiol group reagents, immunotoxins and 276—277
Thiol groups 104 106 107 113 121 148 156 161 162 166—168 171 174 177 180 182 185 see
Thiol groups in immunoglobulins 250—251
Thiol groups, aryl halides and 35
Thiol groups, cleavage and 63 64
Thiol groups, conversion to amines 23 25
Thiol groups, conversion to carboxylic acids 23
Thiol groups, maleimides and 30
Thiol-disulfide interchange 306
Thiolation 17 19 20—22 24 see
Thiophosgene 27 302
Thiopyridine 51
Three-step reactions 210
Threonine 8 12—14 17 23
THS (3,4,5,6-Tetrahydroxysuberimidate) 80
Thymidine-p-aminophenylphosphate 25
Thyrotropin-releasing hormone (TRH) 273
TLCK (p-Toluenesulfonyllysinechloromethyl ketone) 42 44
TMP (4,5',8-Trimethylpsoralen) 319
Toluene diisocyanate 254 259 310
Toluene-2,4-diisocyanate 87 210
Toluene-2-isocyanate-4-isothiocyanate 87
Tosyl chloride (toluenesulfonyl chloride) 23 26
TPCK (p-Toluenesulfonylphenylalaninechloromethyl ketone) 42 44
trans-Diamminedichloroplatinum (II) 321
Transferrin 269 271
Transglutaminase 133 185 203—204
Transition metal ions 309
Traut's reagent 19 20
Tresyl chloride 305 306
TRH (Thyrotropin-releasing hormone) 273
Tri(1-(2-methylaziridenyI))-phosphine oxide 118
Tri(2-chloroemyl)amine (TCEA) 115
Triethylenetetraminehexaacetate 321
Trifluoroacetic acid (TFA) 20
Trifluoromethanesulfonic acid (TFMS)-thioanisole 20
Trimesyl-tric- -alanylazide (TTA) 92 100 -alanylazide (TTA) 92 100
Trimethylolpropane polyglycidyl ether 122
Trinitrobenzenesulfonate 35 36
Tropomyosin 226
Troponin 226
Trypsin 309
Tryptophan 7 8 10 11 40
Tryptophan reagents 42—44
TTA (Trimesyl-tris- -alanylazide) 92 100 -alanylazide) 92 100
Two-step procedures 209—210 252—254 260 267
Tyrosine group reagents 40 41
Tyrosine groups 7 8 10 11 17 see
Tyrosine groups, acylation and 37
Tyrosine groups, conversion to aminotyrosine 25 27
Tyrosine groups, electrophilic reactions and 58
Tyrosine groups, pH effect on 13 14
Tyrosine groups, tetranitromethane and 128
Ultracentrifugation 214
Urease 309
Urokinase-type plasminogen activator 259
Valine 7
Vasopressin 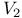 receptors 236 receptors 236
Vicinal dicarbonyl reagents 60
Vicinal diones 128
Vicinal glycol bond cleavage 63 66
Vinyl-benzyl chloride 296
Vinylsulfone 308
Woodward's reagent K 195 197 199—200 204
Woodward's reagent K in small molecule cross-linking 321
Woodward's reagent K, protein immobilization and 303 305
Xanthine oxidase 204
Zero-length cross-linkers 1 123 195—204
Zero-length cross-linkers for carbohydrate activation 203
Zero-length cross-linkers for disulfide bond formation 203
Zero-length cross-linkers in protein immobilization 303—306
Zero-length cross-linkers, carboxyl groups as see under Carboxyl group reagents
Zero-length cross-linkers, enzymes as 203—204
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



 -methyl benzyl thiosulfate
-methyl benzyl thiosulfate -alanylazide) (TDAA)
-alanylazide) (TDAA) 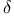 -aminovalerylazide (TDVA)
-aminovalerylazide (TDVA)  -aminobutyrylazide (TDBA)
-aminobutyrylazide (TDBA) 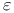 -aminocaproylazide (TDCA)
-aminocaproylazide (TDCA) 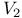 receptors
receptors