|
|
 |
| јвторизаци€ |
|
|
 |
| ѕоиск по указател€м |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Bransden B., Joachain C. Ч Physics of Atoms and Molecules |
|
|
 |
| ѕредметный указатель |
Scattering of atoms by atoms, elastic 534Ч9
Scattering of atoms by atoms, excitation at high velocities 549Ч52
Scattering of atoms by atoms, excitation of heavy ions 547Ч9
Scattering of atoms by atoms, impact parameter method 540Ч3
Scattering of atoms by atoms, inelastic 540Ч52
Scattering of atoms by atoms, merging beam experiment 527
Scattering of atoms by atoms, slow collisions 543Ч7
Scattering of particles, and the optical theorem 468 496
Scattering of particles, Born approximation for 488Ч93
Scattering of particles, boundary conditions 465Ч7 470 485-7
Scattering of particles, by a complex potential 494Ч6
Scattering of particles, by a Coulomb potential 26 492Ч3
Scattering of particles, by a hard sphere 479Ч80
Scattering of particles, by a potential 465Ч93
Scattering of particles, by a square well 477Ч9
Scattering of particles, by a Yukawa potential 491Ч2
Scattering of particles, classical theory of 532Ч9 593-9
Scattering of particles, cross-section 467Ч8
Scattering of particles, general properties 461Ч5
Scattering of particles, integral equation for 484Ч8
Scattering of particles, near a resonance 480Ч4
Scattering of particles, of an atom by an atom see Scattering of atoms by atoms
Scattering of particles, of an electron by an atom see electron scattering by atoms
Scattering of particles, partial wave expansion for 468Ч75
Scattering of radiation see Raman scattering Rayleigh
Scattering, classical 532Ч9 593-9
Scattering, potential 465Ч93
Schr dinger equation, for dinger equation, for 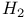 406 406
Schr dinger equation, for dinger equation, for 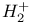 399 404 399 404
Schr dinger equation, for a charged particle in an electromagnetic field 159Ч60 dinger equation, for a charged particle in an electromagnetic field 159Ч60
Schr dinger equation, for a diatomic molecule 386 dinger equation, for a diatomic molecule 386
Schr dinger equation, for a free particle 99 dinger equation, for a free particle 99
Schr dinger equation, for a many-electron atom 291Ч2 dinger equation, for a many-electron atom 291Ч2
Schr dinger equation, for a one-electron atom 128Ч30 dinger equation, for a one-electron atom 128Ч30
Schr dinger equation, for a rigid rotator 91 dinger equation, for a rigid rotator 91
Schr dinger equation, for a several particle system 101Ч2 dinger equation, for a several particle system 101Ч2
Schr dinger equation, for a two-body system 102Ч4 dinger equation, for a two-body system 102Ч4
Schr dinger equation, for a two-electron atom 249Ч50 dinger equation, for a two-electron atom 249Ч50
Schr dinger equation, for an atom in a magnetic field 208Ч10 dinger equation, for an atom in a magnetic field 208Ч10
Schr dinger equation, for an electron in a magnetic field 555 dinger equation, for an electron in a magnetic field 555
Schr dinger equation, for central forces 97 dinger equation, for central forces 97
Schr dinger equation, for electron-atom scattering 501 dinger equation, for electron-atom scattering 501
Schr dinger equation, for potential scattering 465 dinger equation, for potential scattering 465
Schr dinger equation, for the infinite square well 75 dinger equation, for the infinite square well 75
Schr dinger equation, for the linear harmonic oscillator 78 dinger equation, for the linear harmonic oscillator 78
Schr dinger equation, general solution of 67Ч8 dinger equation, general solution of 67Ч8
Schr dinger equation, in momentum space 622 dinger equation, in momentum space 622
Schr dinger equation, relativistic 631Ч2 dinger equation, relativistic 631Ч2
Schr dinger equation, time-dependent 61 dinger equation, time-dependent 61
Schr dinger equation, time-dependent for atom-atom scattering 542 dinger equation, time-dependent for atom-atom scattering 542
Schr dinger equation, time-independent 63Ч4 dinger equation, time-independent 63Ч4
Schr dinger, E. 38 53 dinger, E. 38 53
Schwartz, C. 278
Schwinger, J. 231
Selection rules, for allowed transitions 136 170Ч3
Selection rules, for electric quadrupole radiation 179
Selection rules, for magnetic dipole radiation 178Ч9
Selection rules, in many-electron atoms 355Ч8
Selection rules, in molecular transitions 431Ч6 440-1
Selection rules, in one-electron atoms 136 170Ч3 178-9
Selection rules, in Raman scattering 438
Self-adjoint operators 65
Self-consistent field model see Hartree Ч Fock approximation and Hartree equations
Separated atom limit 412Ч13
Shadow scattering 480
Shape resonance 523
Shell 299Ч300
Shell, closed shell 300
Shell, notation for 300
Shell, subshell 299Ч300 305-6
Shyn, T. 232
Sigma$ lines 211 213Ч14 223-4
Sigma$ quantum number 448
Silverman, S.M. 522
Slater determinant 296Ч8 320Ч1 322-39 414
Slater determinant, parity of 296
Slater, J.C. 292 320
Small angle scattering, classical theory 536Ч7
Sommerfeld, A. 38
Space quantisation 44
Specific charge 5
Spectra see Absorption spectra emission line X-ray band vibrational rotational
Spectra spectral classes of stars 585
Spectral distribution, and PlanckТs law 14
Spectral distribution, and the Rayleigh Ч Jeans law 12
Spectral distribution, for a black body 10Ч15
Spectral terms 29
Spectral terms, and multiplicity 256 397
Spectral terms, diagram of 180
Spectral terms, displaced 371
Spectral terms, identification of 368Ч71
Spectral terms, in L Ч S coupling 342Ч6
Spectral terms, notation 201Ч2 255Ч6 301 395-7
Spectral terms, X-ray terms 380Ч1
Spectroscopic notation 89Ч90 135 201Ч2 255-6
Spectroscopic notation, for molecules 395 449
Spectrum of a Hamiltonian 64Ч6
Spectrum of alkali metals 359Ч64
Spectrum of alkaline earths 368
Spectrum of atomic hvdrogen 28Ч9 33 135Ч6 179Ч80 242
Spectrum of one-electron atoms 179Ч80 203Ч7 241-7
Spectrum of the sun 28 363 584
Spectrum of two-electron atoms 255Ч8
Spherical Bessel functions 100 470Ч1 615
Spherical components of a vector 170 619
Spherical harmonics 84 86Ч8 614-5
Spherical harmonics, addition theorem for 268 615
Spherical harmonics, in real form 88Ч90
Spherical harmonics, parity of 99
Spherical Neumann functions 470Ч1 615
Spherical top 421
Spherical waves 100 466Ч7
Spin angular momentum 44Ч5 91-6
Spin angular momentum, matrix representation for 93
Spin angular momentum, of a molecule 397
Spin angular momentum, of a nucleus 94 233Ч4
Spin wave function for 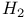 406 406
Spin wave function for two-electron atoms 251Ч4
Spin-orbit coupling 196 198Ч200 209 340 363Ч4 366Ч8 448Ч52 641
Spin-orbitals 197 295Ч7
Spin-orbitals, for Be 333Ч4
Spin-orbitals, for diatomic molecules 413Ч14
Spin-orbitals, for Ne 335Ч6
Spin-orbitals, properties of 330Ч3
Spin-spin coupling 366Ч8
Spinors 93
Spontaneous emission 155 164Ч6
Spontaneous emission, by many-electron atoms 355Ч8
Spontaneous emission, in the dipole approximation 168 186
Spontaneous emission, transition rate for 166
Stark broadening 229
Stark effect 219
Stark effect, and field ionisation 227Ч9
Stark effect, and parity mixing 221Ч5 379
Stark effect, linear 220Ч4
Stark effect, quadratic 225Ч7 377-9
Stark, J. 219
Static-exchange approximation 505Ч7
Stefan Ч Boltzmann law 9 10
Stefan, J. 9
StefanТs law 9
Stellar abundances 586
| Stellar spectra 584Ч8
Stellar spectra, and spectral distribution 586Ч7
Stern Ч Gerlach experiment 42Ч3 554 558
Stern, O. 40
Stimulated emission 155 163Ч4
Stimulated emission, and the laser 562Ч4
Stimulated emission, and the maser 568
Stimulated emission, by many-electron atoms 355Ч8
Stimulated emission, in the dipole approximation 168
Stimulated emission, transition rate for 163Ч4
Stoletov, M. 15
Stoney, C.I. 4 6
Stopping power 518Ч19
Strong interaction 151
Sum rule for line intensities 369Ч70
Sum rule for oscillator strengths 181Ч2
Superposition principle 62
Symmetrical top 421 430Ч1
Symmetrisation postulate 106
Terms see Spectral terms
Thermal broadening 588
Thomas Ч Fermi model 293 313Ч20
Thomas Ч Fermi model, Thomas Ч Fermi equation 315
Thomas Ч Reiche Ч Kuhn sum rule 181Ч2
Thomson scattering 18 19
Thomson, Sir J.J. 4 5 6 15 18 26
thresholds 463Ч4
Thresholds, anomalous behaviour at 523
Time reversal invariance 233
Tomonaga, S. 231
Total angular momentum 42 46 95Ч6 104
Townes, C.H. 567
Townsend, J.S. 4
Transition probability 113Ч16
Transition rate 116
Transition rate, for an atom in an electromagnetic field 160Ч70
Trial functions 119
Tritium 149 574Ч5
Trivalent elements 368
Two-centre integrals 645Ч6
Two-electron atoms, and Slater determinants 297
Two-electron atoms, and the variational method 271Ч7 283-6
Two-electron atoms, doubly excited states of 286Ч8 522-5
Two-electron atoms, energy spectrum of 255Ч8
Two-electron atoms, excited states of 278Ч86
Two-electron atoms, fine structure of 366Ч8
Two-electron atoms, ground state energy of 267Ч78
Two-electron atoms, independent particle model for 258Ч67
Two-electron atoms, level scheme of 255Ч8
Two-electron atoms, para and ortho states of 249Ч51 255
Two-electron atoms, perturbation theory of 267Ч71 276-7 280Ч3
Two-electron atoms, Schr dinger equation for 249Ч50 dinger equation for 249Ч50
Two-electron atoms, spectra of 364Ч8
Two-electron atoms, spin wave functions for 251Ч4
Two-electron atoms, two-photon transitions 183 230 572
Uhlenbeck, G.E. 44
Ultraviolet spectra 28 286Ч7 384 438
Ultraviolet spectra, in astronomy 584
Uncertainties 61
Uncertainty principle see Heisenberg uncertainty principle
Ungerade states 397 399Ч415
Unitary matrix 70
Unitary transformations 71Ч2
United atom limit 412Ч13
Valence bond method see Heitler-London method
valence electrons 301 383 412
Valency 414Ч5
Van der Waals interaction 532
Variation of constants 111Ч16
Variation-perturbation method 122Ч3 276-7
Variational method 116Ч22
Variational method, and the Hartree Ч Fock method 320Ч5
Variational method, for 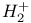 400Ч4 400Ч4
Variational method, for 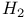 405Ч11 405Ч11
Variational method, for excited states 119Ч22
Variational method, for scattering of 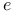 by by 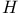 506 506
Variational method, for two-electron atoms 271Ч7 283-6
Vector model for angular momentum 45Ч6 88 94
Vector operators 94 216Ч17 357 618Ч20
Vibrational motion of a molecule 384Ч6 389Ч94 420-1
Vibrational-rotational spectra 432Ч6 440-4
Virial theorem 147Ч8
Visible spectra 28Ч9 359Ч71 438-44
Visible spectra, in astrophysics 583Ч7
Volume effect 151 232
von Laue, M. 19
von Neumann Ч Wigner non-crossing rule see Non-crossing rule
Water molecule 421 422Ч3
Wave function, electronic, for diatomic molecule 388 394Ч420
Wave function, expansions of 66Ч67
Wave function, for 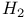 406Ч11 (see also Orbitals spin-orbitals) 406Ч11 (see also Orbitals spin-orbitals)
Wave function, for a free particle 54 99Ч101
Wave function, for a several-particle system 101Ч2
Wave function, for a spin one-half particle 94
Wave function, for a two-body system 102Ч4
Wave function, for one-electron atoms 136Ч45
Wave function, for the infinite square well potential 74Ч76
Wave function, for the linear harmonic oscillator 78Ч82
Wave function, for the rigid rotator 90Ч91
Wave function, for two-electron atoms 250Ч5 259Ч67 271Ч5 284Ч6 297
Wave function, Heitler Ч London 408Ч11
Wave function, in momentum space 55 57 621
Wave function, in momentum space for one-electron atoms 621Ч8
Wave function, interpretation of 58Ч59
Wave function, molecular 431 452
Wave function, normalisation of 58Ч60
Wave function, of a many-electron system 291Ч2 296
Wave function, of a particle in a box 74Ч6 308
Wave function, of orbital angular momentum 83Ч90
Wave function, orthogonality of 65Ч66
Wave function, symmetry of 104Ч6
Wave function, variational for 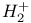 400Ч4 400Ч4
Wave number, definition of 28
Wave packets 55Ч7
Weak interaction 151 178 222
Welton, T.A. 231
Wheeler, J.A. 150
Wien, W. 10 11
WienТs displacement law 10 586
Wigner Ч Eckart theorem 217 357 619
Williams, J.F. 501
Williams, N.H. 459
Willis, B.A. 501
Wilson, C.T.R. 22
Wilson, H.A. 7
Wilson, W. 38
Wollaston, W.H. 584
X-ray scattering factor 508
X-rays, and the dipole approximation 166
X-rays, discovery of 18
X-rays, in astrophysics 584
X-rays, scattering of 18Ч22
X-rays, spectra 38Ч40 379Ч81
Zeeman effect 44 195 207Ч19 374Ч7 571 588
Zeeman effect, and magnetic resonance 558
Zeeman effect, and polarisation 212Ч14
Zeeman effect, and the Paschen Ч Back effect 214Ч5 375
Zeeman effect, anomalous 215Ч19 375
Zeeman effect, for strong fields 210Ч14 248
Zeeman effect, in hyperfine structure 245 376Ч7
Zeeman effect, normal 211
Zeeman, P. 207
zero point energy 76 79 231 392
|
|
 |
| –еклама |
 |
|
|
 |
|
ќ проекте
|
|
ќ проекте



 dinger equation, for
dinger equation, for 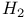
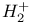
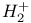
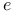 by
by