|
|
 |
| јвторизаци€ |
|
|
 |
| ѕоиск по указател€м |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Bransden B., Joachain C. Ч Physics of Atoms and Molecules |
|
|
 |
| ѕредметный указатель |
Electromagnetic radiation, energy density of 157Ч8
Electromagnetic radiation, polarisation of 157
Electromagnetic radiation, pulse of 158
Electromagnetic radiation, transverse nature of 156
Electromagnetic spectrum 19
Electron 3Ч8
Electron affinity 306
Electron configuration 265 298Ч300
Electron diffraction 47Ч9
Electron gas 308Ч13
Electron gun 499
Electron scattering by atoms, and ionisation 519Ч21
Electron scattering by atoms, and resonance phenomena 522Ч5
Electron scattering by atoms, and the Bethe approximation 518
Electron scattering by atoms, and the optical potential 511Ч13
Electron scattering by atoms, and the two-state approximation 513
Electron scattering by atoms, at high energies 507Ч10 514Ч19
Electron scattering by atoms, Born approximation for 507Ч10 514Ч19 520-1
Electron scattering by atoms, by the positive helium ion 523Ч4
Electron scattering by atoms, close coupling approximation for 510Ч11
Electron scattering by atoms, experimental arrangement for 499Ч501
Electron scattering by atoms, static exchange approximation for 505Ч7
Electron scattering by atoms, theoretical principles of 501Ч5
Electron spin 44Ч5 91Ч4 173
Electron spin resonance see Paramagnetic resonance
Electron, charge of 7Ч8
Electron, discovery of 4Ч8
Electron, mass of 8
Electronic band spectra see Band spectra
Electronic energy of a diatomic molecule, general form of 389Ч90
Electronic spectra of molecules 438Ч47
Electronic spectra of molecules, and nuclear spin 452Ч5
Electronic wave equation 388
Emission of radiation 155 163Ч6
Emission of radiation, emission spectra 27 362 438Ч9 584
Empedocles 1
Energy level spectrum, and the Franck Ч Hertz experiment 36
Energy level spectrum, eigenvalues spectrum of 64Ч6 (see also Hamiltonian operator)
Energy level spectrum, energy operator 55
Energy level spectrum, of a free particle 100
Energy level spectrum, of a linear harmonic oscillator 79
Energy level spectrum, of a molecule 383Ч6
Energy level spectrum, of a rigid rotator 91
Energy level spectrum, of alkali metals 359Ч64
Energy level spectrum, of an electron gas 308Ч13
Energy level spectrum, of an infinite square well potential 74Ч6
Energy level spectrum, of one-electron atoms 133Ч6 197Ч203 242
Energy level spectrum, of one-electron atoms in the Bohr model 32
Energy level spectrum, of two-electron atoms 255Ч8
Energy, and the uncertainty principle 57Ч8
Equilibrium distance in a diatomic molecule 389Ч94 passim
Equivalent electrons 299Ч300 344Ч6 351
Exchange degeneracy 259 280 295
Exchange force 266
Exchange integral 281 324 409 415
Exchange potential 327 507 511
Exclusion principle see Pauli exclusion principle
Exotic atoms 150 247
expectation values 60Ч1
Expectation values, of 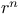 in hydrogen 145Ч6 in hydrogen 145Ч6
Expectation values, time variation of 63
Fabry, C. 232
Fano Ч Lichten model 548
Faraday cup 501
Faraday, M. 3
FaradayТs constant 3
FaradayТs laws of electrolysis 3
Fermi contact interaction 238
Fermi electron gas see Electron gas
Fermi energy 310
Fermi sphere 312Ч13
Fermi surface 313
Fermi Ч Dirac statistics 106 233
fermions 106 233 452
FermiТs Golden Rule 116 165 286
Feynman, R.P. 231
Fine structure constant 33 670
Fine structure in alkali spectra 363Ч4
Fine structure multiplets 201Ч7 346-9
Fine structure multiplets, inverted multiplets 349
Fine structure multiplets, regular multiplets 349
Fine structure of many-electron atoms 346Ч52
Fine structure of one-electron atoms 195Ч207
Finite nuclear mass, correction for, in the Bohr model 34Ч6
Fluorescence 444Ч5
Flux of particle beam 464 466 595
Fock, V. 320 626
For spin 93
Forbidden transitions 167
Form factor for atoms 508 515
Fortrat parabola 441Ч2
Franck Ч Condon factor 444
Franck Ч Condon principle 442Ч4
Franck Ч Hertz Experiment 36Ч8
Franck. J. 36
Frankowski, K. 278
Fraunhofer lines 584
Fraunhofer, J. 28 584
Free particle 99Ч101
Free particle, relativistic 631Ч2 633-5
Fundamental series 362
Gauge invariance 156
Gay Ч Lussac, J.L. 2
Gay Ч LussacТs law 2
Geiger, H. 22 23 24 26 40
Generalised oscillator strength 516Ч18
Generating functions, for associated Laguerre polynomials 138
Generating functions, for Gegenbauer polynomials 626
Generating functions, for Hermite polynomials 81 608
Generating functions, for Laguerre polynomials 136 610
Generating functions, for Legendre polynomials 84
Generating functions, gerade states 397 399Ч415
Gerlach, W. 40
Germer, L.H. 47
Golden Rule 116 165 286
Goudsmit, S. 44
GreenТs function for a free particle 484Ч8
Grotrian diagram for beryllium 372Ч3
Grotrian diagram for calcium 365
Grotrian diagram for helium 365
Grotrian diagram for hydrogen 180
Grotrian diagram for lithium 360
Grotrian diagram for sodium 361
Grotrian, W. 229
gyromagnetic ratio 42 44 209 639
H nsch, T.W. 572 nsch, T.W. 572
Hadronic atoms 151Ч2
Half-life see Lifetime
Hallwachs, W. 15
Halogens 306 418Ч9
Hamiltonian operator 61 64Ч5
Hamiltonian operator, for a rigid rotator 91
Hamiltonian operator, for a several particle system 101
Hamiltonian operator, for a two-particle system 102Ч3
Hamiltonian operator, for an atom in a magnetic field 207Ч10
Hamiltonian operator, for central potentials 97
Hamiltonian operator, for charged particles 158Ч60
Hamiltonian operator, for many-electron atoms 291Ч4 339Ч41
Hamiltonian operator, for one-electron atoms 129
Hamiltonian operator, for two-electron atoms 250
Hamiltonian operator, with relativistic corrections 196 633 635 641
Hamiltonian, classical 101
Hamiltonian, classical, for a charged particle in an electromagnetic field 629Ч30
Hartree equations 337Ч8
Hartree Ч Fock approximation 320Ч37
Hartree Ч Fock approximation, and KoopmanТs theorem 330
Hartree Ч Fock approximation, for Be 333Ч5
Hartree Ч Fock approximation, for Ne 335Ч7
Hartree Ч Fock approximation, Hartree Ч Fock equations 325Ч7
Hartree Ч Fock approximation, self-consistent field 328
| Hartree, D.R. 292 320
Harvard classification 585
HCL molecule 385 418 431Ч2 435
Heisenberg equations of motion 72 166
Heisenberg uncertainty principle 56Ч8 124 184 187 384 484 532 648-9
Heisenberg uncertainty principle, and zero-point energy 76 79
Heisenberg, W 38 53 58 282
Heitler Ч London method 408Ч10
Heitler, W. 408
Helicity 176
Helium atom see Two-electron atoms
Helium atom, discovery of 584
Helmholtz, H. 3 4
Hermite polynomials 79 81 608Ч10
Hermitian operators 65
Hertz, G. 36
Hertz, H. 15
Herzberg, G. 278
Homonuclear molecules 396Ч7 412Ч14 431 452Ч5
Hund Ч Mulliken method see Molecular orbitals
HundТs cases of angular momentum coupling 448Ч52
HundТs rules 346 348
Hybrid orbital 417Ч18 423-5
Hybridisation 418 423Ч5
Hydrides 417Ч8
Hydrogen atom see One-electron atoms
Hydrogen concentration in galaxy 588Ч9
Hydrogen isotopes 35 149
Hydrogen molecular ion, structure of 399Ч405 412Ч13
hydrogen molecule 106 383 438 452Ч5
Hydrogen molecule, structure of 405Ч11
Hydrogenic ions 36 128 149 183
Hylleraas trial functions 273Ч4
Hylleraas Ч Undheim theorem 122 285
Hylleraas, E.A. 273 284
Hyperfine structure 134 232Ч45 371-4
Hyperfine structure, and the Zeeman effect 245 376Ч7
Hyperfine structure, hyperfine structure constant 372
Hyperonic atoms 152
Identical nuclei and molecular spectra 452Ч5
Identification of terms 368Ч71
Impact parameter 474Ч5 594
Independent particle model 258Ч67 292 320
Indistinguishable particles 104Ч6
Inelastic cross-section 495
Inelastic cross-section, for atom-atom scattering 550
Inelastic cross-section, for electron scattering 513Ч18
Inelastic scattering 462 513Ч18 527Ч8 549-52
Infinite square well 74Ч6
Infra-red spectra 28Ч9 385Ч6 432-5
Infra-red spectra, in astronomy 583
Integrals containing hydrogenic wave functions 610Ч11
Integrals containing oscillator wave functions 608Ч10
Integrals containing spherical harmonics 618
Intensities see Line intensities
Interchange operator 104Ч6
Intercombination lines 255 366
Intermediate coupling 341
Interstellar medium 589
Intersystem crossing 446
Interval rules 241 349 368 373
Inversion spectrum of 455Ч9 567
Inversion spectrum of ammonia 455Ч9
Ion-atom collisions see Scattering of atoms by atoms
Ionic bonding 408 418Ч20
Ionisation 464
Ionisation potential 32 148Ч53 302Ч6 411
Ionisation, by electron impact 519Ч21
Iso-electronic sequence 362
Isotopes 27
Isotopes, of hydrogen 35 149
Isotopic shift 35 232 245Ч7
Jansky, K. 583
Jeans, Sir J. 11
Joachain, C.J. 275 285
Jordan, P. 53
K series 39 380
K shell 40 139 300
Kaonic atom 152
Kinematics of scattering 600Ч7
Kinetic energy, relativistic corrections to 196Ч8 641
Kinetic theory of gases 1 2
Kinoshita, T. 274
Kirchhoff, G.R. 9 27 584
Klein Ч Gordon equation 631Ч2
KoopmanТs theorem 330
L series 39 380
L shell 40 139 300
L Ч S coupling see Russell Ч Saunders coupling
Laboratory system of coordinates 463 465 600Ч7
Lagrange multipliers 118 324
Laguerre polynomials 136Ч8
Lamb shift 134 195 207 229Ч32 572
Lamb Ч Retherford experiment 229Ч32
Lamb, W.E. 207 229Ч32
Lambda$ quantum number 395
Lambda$-doubling 396 451Ч2
Land  factor 217 234Ч5 243 375-6 factor 217 234Ч5 243 375-6
Land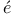 interval rule see Interval rule interval rule see Interval rule
Landau levels 248
LaporteТs rule 358
Larmor angular frequency 42
Larmor frequency 211 555
Lasers 155 158 164 562Ч7
Lasers, and spectroscopy 571Ч2
Lasers, gas 565
Lasers, ruby 565
Lasers, tunable 571
Lassettre, E.N. 522
Lawson criterion 576Ч9
LCAO Method 400 422
Legendre polynomials 84Ч6 615
Lenard, P. 5 15
Lennard Ч Jones potential 532 535
Leptons 149
Leucippus 1
Level shifts 525
Level widths 183Ч7 523-5
Level widths, natural width 187
LevinsonТs theorem 479
Lifetimes of atomic levels 183
Lifetimes of atomic levels, and level widths 184Ч7
Lih molecule 417Ч18
Line broadening 187Ч9 229 572 587
Line intensities 180Ч2 205-7
Line intensities, and the identification of terms 369Ч71
Line shapes 183Ч6 588
Line spectra of atoms 27Ч9
Line widths 183Ч9
Linear harmonic oscillator 76Ч82
Linear harmonic oscillator, and the virial theorem 148
Lo Surdo, A. 219
Logarithmic derivatives 476
London, F. 408
Long-range interaction between atoms 528Ч32
Lorentz triplet 211
Lorentzian distribution 185Ч8 483
Loschmidt, J. 2
Lummer, O. 10
Lyman series 28 204
M shell 139 300
Madden, R.B. 287
Magnetic broadening 588
Magnetic dipole moment, and magnetic resonance 554Ч61
Magnetic dipole moment, and spin 44 209
Magnetic dipole moment, anomalous 243
Magnetic dipole moment, in the Bohr model 40Ч1
Magnetic dipole moment, of the nucleus 233Ч5
Magnetic dipole moment, orbital 41 209
Magnetic dipole transition 167 178Ч9
|
|
 |
| –еклама |
 |
|
|
 |
|
ќ проекте
|
|
ќ проекте



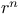 in hydrogen
in hydrogen 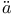 nsch, T.W.
nsch, T.W. 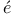
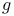 factor
factor