|
|
 |
| јвторизаци€ |
|
|
 |
| ѕоиск по указател€м |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Bransden B., Joachain C. Ч Physics of Atoms and Molecules |
|
|
 |
| ѕредметный указатель |
magnetic quantum number 84 134
Magnetic resonance see Paramagnetic resonance nuclear
Magnetic resonance magneton see Bohr magneton nuclear
Magneton many-electron atoms 290
Many electron atoms, and ionisation potentials 302Ч7
Many electron atoms, and the central field approximation 292Ч300
Many electron atoms, and the periodic table 300Ч7
Many electron atoms, corrections to the central field model 239Ч52
Many electron atoms, effective potential in 292Ч5
Many electron atoms, fine structure of 346Ч52
Many electron atoms, Hartree Ч Fock model of 320Ч37
Many electron atoms, hyperfine structure of 371Ч4
Many electron atoms, Schr dinger equation for 291Ч2 dinger equation for 291Ч2
Many electron atoms, the Stark effect in 377Ч9
Many electron atoms, the Zeeman effect in 374Ч7
Many electron atoms, Thomas Ч Fermi model of 313Ч20
Marsden, E. 23 24 26 40
Maser 164 243 459
Maser, ammonia 567Ч71
Mass polarisation 250 277 643
Matrix elements of operators 67 69Ч71
Matrix representation of angular momentum operators 612Ч14
Matrix representations 69Ч71
Matrix, diagonal 71
Matrix, equations of motion 72
Matrix, Hermitian, see Hermitian operators
Matrix, unitary 70
Maxwell, J.C. 1 9 155Ч6
Melvill, Th 27
Mendeleev, D.I. 39 306
Meson 151
Metastable levels 183
Metastable levels, quenching of 224Ч5
Metastable state 484
Methane molecule 383Ч4 421 423
Michelson, A. 232
Microwave spectra 386 432
Millikan, R.A. 7 17 18
MillikanТs oil drop experiment 7Ч8
Minimum principle for the energy 119
Mole 2
Molecular orbitals 405
Molecular orbitals, and atom-atom scattering 543Ч9
Molecular orbitals, for 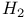 405Ч8 405Ч8
Molecular orbitals, for heteronuclear molecules 415Ч20
Molecular orbitals, for homonuclear molecules 412Ч14
Molecular orbitals, notation for 413 416
Molecular structure 383
Molecular structure, general nature of 383Ч6 (see also Diatomic molecule polyatomic
Molecules see Diatomic molecule polyatomic
Moment of inertia of a molecule 385 430
Momentum and the uncertainty principle 57Ч8
Momentum operator 55
Momentum space wave functions 55 57
Momentum space wave functions, hydrogenic 621Ч8
Momentum transfer 490 515
Morse potential 391Ч4
Morse, P.M. 391
Moseley, H. 38 39
MoseleyТs law 39
MoseleyТs plot 39
Multiplet see Fine structure hyperfine Stark Zeeman
Multiplicities see Spectral terms
Multipole moments 233 (see also Electric dipole moment electric magnetic
Muon 149Ч50 247
Muonic atoms 150Ч1 247
Muonium 149
Nacl molecule 418Ч20
Negative hydrogen ion 249 257Ч8 260 270 273 275
Neon ground state 335Ч7
Neutron 26Ч7
Neutron stars 248
Newton, Sir I. 9 27
NO molecule 397
Noble gases 306
Non-crossing rule 398Ч9 412
Non-linear optics 572
Non-localised bonds 425Ч6
Normal modes 421
Normalisation of wave functions 58Ч60 66
Nuclear magnetic resonance 560Ч1
Nuclear magneton 234
Nuclear moments 233Ч4
Nuclear spin 94 233Ч45 452Ч5
Nuclear wave equation 388
Nucleus, discovery of 23Ч6
Nucleus, size of 26
Nucleus, spin of 233Ч4
Observables 65
Observables, commuting 72Ч3
Old quantum theory 38 136
One-electron atoms, and expectation values of 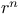 145Ч7 145Ч7
One-electron atoms, and parity 144Ч5
One-electron atoms, and the Stark effect 219Ч27
One-electron atoms, and the Zeeman effect 207Ч19
One-electron atoms, energy levels of 133Ч6
One-electron atoms, field ionisation of 227Ч9
One-electron atoms, fine structure in 201Ч7
One-electron atoms, hyperfine structure of 232Ч45
One-electron atoms, in the Bohr model 29Ч36
One-electron atoms, photoionisation of 189Ч93
One-electron atoms, radial distribution function for 142Ч4
One-electron atoms, Schr dinger equation for 128Ч33 dinger equation for 128Ч33
One-electron atoms, spectrum of 133Ч6 179Ч80
One-electron atoms, wave functions for 131Ч3 136Ч41
One-electron atoms, wave functions in momentum space 621Ч8
Operators in quantum mechanics 55 61 63 64Ч5 66Ч73
Oppenheimer, J. 228
Optical potential 511Ч13
Optical theorem 468 474 496
Orbital angular momentum 82Ч4 87Ч8 614
Orbital angular momentum, commutation relations 82
Orbital angular momentum, eigenfunctions of 84Ч90 614
Orbital angular momentum, in polar coordinates 83Ч4
Orbital angular momentum, operators for 82 614
Orbital angular momentum, quantum number 84
Orbital angular momentum, raising and lowering operators 87 614
Orbital angular momentum, spectroscopic notation for 90
Orbitals 141 264 294
Ornstein, Burger and Dorgelo sum rule 369Ч70
Ortho hydrogen 454
Orthogonality relations, for associated Legendre functions 85
Orthogonality relations, for Legendre polynomials 85 (see also Orthonormality)
Orthonormality 60 66
Orthonormality, for harmonic oscillator wave functions 81
Orthonormality, for spherical harmonics 86 614
Oscillator strengths 181Ч2
Oscillator strengths, generalised 516Ч18
Oxygen molecule 383 397 414 438 452Ч3
P-branch 433Ч5 441
Pairing 414Ч15
Para hydrogen 454
Paramagnetic resonance 554Ч61
Paramagnetic resonance, in solids 559Ч61
Paramagnetic resonance, Rabi apparatus for 558Ч9
Paramagnetic resonance, Rabi formula for 558
Paramagnetism 554
Parity 76 82 98Ч9 144Ч5 171 178 202 220Ч2 233 296 358 ungerade
Partial wave cross-section 473
Partial wave expansion 468Ч75 488
Paschen series 29 205
Paschen Ч Back effect 214Ч15 375
Pauli equation 638Ч9
Pauli exclusion principle, and antisymmetry 106 251 254Ч5 291 296 299 307 310 406 414 415 452
Pauli spin matrices 94 635
Pauli, W. 233 246 296
Peierls, Sir R.E. 246
Pekeris, C.L. 278 284 285
Periodic system 300Ч7
Periodic table 39 306Ч7
| Permutation operator 105Ч6 250-1
Perot, A. 232
Perrin, J. 3
Perturbation theory, and corrections to the central field approximation 339Ч41 349Ч51
Perturbation theory, and correlation energy 339
Perturbation theory, and Raman scattering 436Ч8
Perturbation theory, for an atom in an electromagnetic field 160Ч1
Perturbation theory, for fine structure 197Ч201
Perturbation theory, for scattering see Born approximation
Perturbation theory, of atom-atom interactions 529Ч32
Perturbation theory, of hyperfine structure 235Ч40
Perturbation theory, of the Stark effect 219Ч23 225Ч7 377-8
Perturbation theory, of the Zeeman effect 214Ч19 374-7
Perturbation theory, of two-electron atoms 258Ч71 280-3
Perturbation theory, time-dependent 111Ч16
Perturbation theory, time-independent 106Ч11
Pfund series 29
Phase shifts 472Ч7
Phase shifts, absolute definition of 476
Phase shifts, complex 494Ч6
Phase shifts, computation of 476
Phase shifts, for a hard sphere potential 479
Phase shifts, integral equation for 476
Phase shifts, near a resonance 480Ч3
Phosphorescence 446Ч8
photoelectric effect 15Ч17 188Ч93
Photoelectric effect, and MillikanТs experiments 17Ч18
Photoelectric effect, EinsteinТs equation for 17
Photoionisation 188Ч93
photons 15 17 21 155 157 159
Photons, absorption of 164
Photons, and the Compton effect 20Ч2
Photons, and the electromagnetic spectrum 18Ч19
Photons, and the photoelectric effect 17
Photons, emission of 163Ч66
Photons, parity of 178
Photons, spin of 173Ч8
Photosphere 584
Pionic atom 152
Planck, M. 12 29
PlanckТs constant 13 14 670
PlanckТs distribution law 14 169 586
Plane waves 54 100Ч1 156Ч7 466 485 504
Polarisability see Dipole polarisability
Polarisation potential 512Ч13
Polarisation vector 157 173
Polarisation vector, spherical components of 170
Polarisation, circular 174Ч6
Polarisation, in the Zeeman effect 212Ч14
Polarisation, linear 157
Polyatomic molecules, electronic structure of 422Ч6
Polyatomic molecules, rotational motion of 430Ч1
Polyatomic molecules, vibrational motion of 421
Population inversion 564Ч6 568
Positron 149 151 633
Positronium 149
Poynting vector 157
Predissociation 444
Pressure broadening 187 588
principal quantum number 133 295
Principal series 362
Pringsheim, E. 10
Probability amplitudes 66 542Ч6
Probability conservation 62
Probability current density 62 467
probability density 58 141
Proust, J.L. 2
Q-branch 436 441
Quadrupole coupling constant 245
Quantisation of angular momentum 31 44Ч6
Quantisation of the electromagnetic field 17 155
Quantum defects 360Ч2
Quantum theory 53 123
Quantum theory, and photons 17 155
Quantum theory, of Planck 12Ч15
R branch 433Ч5 441
R ntgen, W.K. 18 ntgen, W.K. 18
Rabi 1 1 558
Rabi flopping formula 558 571
Radial density function 336
Radial quantum number 133
Radial Schr dinger equation, for a free particle 99 dinger equation, for a free particle 99
Radial Schr dinger equation, for central potentials 97 dinger equation, for central potentials 97
Radial Schr dinger equation, for nuclear motion 389 429 dinger equation, for nuclear motion 389 429
Radial Schr dinger equation, for one-electron atoms 129 dinger equation, for one-electron atoms 129
Radial Schr dinger equation, for potential scattering 469 dinger equation, for potential scattering 469
Radial Schr dinger equation, in the Hartree Ч Fock method 332Ч5 dinger equation, in the Hartree Ч Fock method 332Ч5
Radial wave functions, for a free particle 100
Radial wave functions, for one-electron atoms 136Ч9
Radial wave functions, for potential scattering 469Ч73
Radiofrequency spectra in astronomy 583
Rainbow angle 535
Rainbow scattering 538Ч9
Raman scattering 436Ч8
Raman, C.V. 436
Rayleigh scattering 436
Rayleigh Ч Jeans distribution law 12
Rayleigh Ч Ritz variational method 119
Rayleigh Ч Schr dinger perturbation theory 106Ч11 dinger perturbation theory 106Ч11
Rayleigh, Lord 11 436
Reaction cross section 495
Reactions 462
Reactions, endothermic 463
Reactions, exothermic 463
Recurrence relations for associated Legendre functions 85
Recurrence relations for Hermite polynomials 81
Recurrence relations for Laguerre polynomials 137
Recurrence relations for Legendre polynomials 85
Reduced mass 26 34 102 129 643
Relativistic corrections see Fine structure
Relativistic wave equations 631Ч41
Representation 69
Resonance lines 36 362Ч3
Resonances in scattering 287Ч8 480-4
Retherford, R.C. 207 229
Rigid rotator 90Ч1 394
Ritz combination principle 29
Ritz, W. 29 361
Robiscoe, R.T. 232
Rotational broadening 588
Rotational constant 391 393 429Ч36 441-2
Rotational energy see Rotational motion of a molecule
Rotational motion of a molecule 384Ч6 428Ч32 448-52
Rotational spectra of diatomic molecules 431Ч2 448Ч52
Rotational-vibrational coupling 394
Rovibronic states 389 433
Russell Ч Saunders coupling 341Ч9
Russell Ч Saunders coupling, and selection rules 358
Russell Ч Saunders coupling, in heavier atoms 368
Russell Ч Saunders coupling, in two-electron atoms 366
Rutherford model of the atom 26Ч7
Rutherford scattering 26 493 597Ч9 607
Rutherford, Lord 23 29
Rydberg atoms 152Ч3
Rydberg constant 28
Rydberg constant, for deuterium 36
Rydberg constant, for hydrogen 28 36
Rydberg constant, for one-electron atoms 36
Saha, N.M. 586
Satellite astronomy 584
Scalar operators 618
Scattering amplitude 467
Scattering amplitude, for a complex potential 495
Scattering amplitude, for Coulomb scattering 493
Scattering amplitude, integral representation for 488
Scattering amplitude, near a resonance 481Ч3
Scattering amplitude, partial wave expansion for 473 495
Scattering length 477Ч8
Scattering of  particles 24Ч6 particles 24Ч6
Scattering of atoms by atoms, classification of processes 527Ч8
|
|
 |
| –еклама |
 |
|
|
 |
|
ќ проекте
|
|
ќ проекте



 dinger equation for
dinger equation for 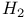
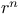
 particles
particles