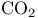|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Planck M. — Treatise on thermodynamics |
|
|
 |
| Предметный указатель |
Abnormal vapour densities 32
Absolute temperature 6
Absolute temperature, deduced from Thomson and Joule’s experiments 135
Acetate silver 250
Acetate silver, sodium 264
Acetic acid 264
Adiabatic process 62 112
Affinity of solution for water 115
Aggregation, states of 71
Aggregation, states of, coexistence of states of 159
Aggregation, states of, systems in different states of 139
Air, composition of 12
Ammonium carbamate, evaporation of 194
Ammonium chloride, evaporation of 194
Andrews 147
apt 15
Arrhenius’ theory of electrolytic dissociation 257
Arrhenius’ theory of isohydric solutions 267
Arrheuius 241 245 207
Atmospheric pressure 4
Atom, definition of 27
Atomic heat 36
Avogadro’s law 26 29 60
Babo’s law 206
Bcrtholet’s principlo 116
Bertholct 15 73 75 250
Binary electrolyte 244
Bodenstein 227
Boiling point, elevation of 208 254 291
Boltzmann 98
Boyle and Gay Lussac 205
Boyle’s law 5 60
Broeusted 279
Byk, A. 179
Calorie, laboratory 35
Calorie, large 35
Calorie, moan 35
Calorie, small 35
Calorie, zero 35
Calorimetric bomb 73
Carbon dioxide, Clausius’ constants for 15
Carbon dioxide, isotherms of 16
Carbon dioxide, van der Waals’ constants for 15
Carbon, combustion of 76
Carnot — Clapeyron equation 149 243
Carnot’s theory 38
Carnot’s theory, cycle 65 109
Characteristic equation 5 6 11
Characteristic equation of water vapour 32
Characteristic equation, deduced from Thomson and Joule’s experiments 133
Characteristic equation, functions 121
Characteristic, constant 11
Chemical constant 280
Clapeyron 149
Clausius 15 90 91 149 283
Clausius’ equation 14 147
Clausius’ equation, form of the second law 99
Clausius’ equation, notation 57
Clausius’ equation, statement of the first and second laws 104
Coefficient of compressibility 8
Coefficient of elasticity 8
Coefficient of expansion 7
Coefficient of expansion at absolute zero 277 286
Coefficient of pressure 8
Coexistence of states of aggregation 159
Combustion, influence of temperature on 78
Combustion, of carbon 76
Combustion, of methane 77
Combustion, of sulphur 77
Condensed system 187
Condition of completo reversibility of a process 97
Condition of completo reversibility of equilibrium 118 143 182
Condition of completo reversibility of equilibrium of a gas mixture 223—225
Conductivity of water, electrical 242
Conservation of energy 42
Constant, chemical 280
Constituents, independent 179
Corresponding, point 167
Corresponding, states 20
Critical, point 18
Critical, point of 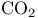 19 19
Critical, pressure 18
Critical, solution temperature 188
Critical, specific volume 18
Critical, temperature 18 158
Cryohydrate 187
Curves, of evaporation 162 167
Curves, of fusion 162 167
Curves, of sublimation 162 167
Cycle, Carnot’s 65 109
Cycle, of operations 46
Dalton 11 21
Davis, H.N. 154
Davy 38
Debye 277
Debye’s law 279
Decrease of free energy by dilution 116
Deductions from the second law of thermodynamics 108
Degree of dissociation of electrolytes from the heat of dissociation 289
Density, abnormal vapour 32
Density, specific 7
Depression of freezing point 209 254 291
Developable surfaces 170
Deviation from Gay Lussac’s law 130
Difference of specific heats 127
Diffusion, increase of entropy by 222
Diffusion, irreversible 222
Dilute solutions, thermodynamical theory of 229—271
Dilute solutions, thermodynamical theory of, energy of 232
Dilute solutions, thermodynamical theory of, entropy of 231
Dilution, decrease of free energy by 115
Dilution, heat of 205
Dilution, infinite 72
Dilution, law of, of binary electrolytes 244
Direction of natural processes 111
Dissipation of energy 104
Dissociation, Arrhenius’ theory of electrolytic 257
Dissociation, at absolute zero 242
Dissociation, graded 227
Dissociation, of 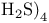 226 226
Dissociation, of hydriodic acid 246
Dissociation, of iodine vapour 227
Dissociation, of water 240
Dissolved substance 204
Distribution law, Nernst’s 262
Divariant system 188
Dulong and Petit’s law 36
Dyne 4
Elasticity, coefficient of 8
Electrical conductivity of water 242
Electrolyte, binary 244
Electrolytic dissociation, Arrhenius’ theory of 257
Elevation of boiling point 208 254 291
Endothertnal process 39
Energetics 81 80
Energy, change of 45
Energy, conservation of 42
Energy, definition of 42
Energy, dissipation of 113
Energy, free 113
Energy, free, of perfect gas 117
Energy, internal 48
Energy, internal, of perfect gas 60
Energy, latent 113
Energy, of a solution 72
Energy, of dilute solution 232
Energy, of gas mixture 216
Energy, potential 47
Energy, total 113
Energy, zero 45
| Entropy, absolute value of 274
Entropy, definition of 100
Entropy, diminution of 100
Entropy, increase of, by diffusion 222
Entropy, maximum value 120
Entropy, of a gas 92
Entropy, of a system of gases 95
Entropy, of dilute solution 233
Entropy, of gas mixture 220—222
Entropy, principle of increase of entropy 103
Entropy, specific 125
Equation, characteristic 5 6 11
Equation, Clausius’ 14 147
Equation, deduced from Thomson and Joules’ Experiments 133
Equation, of water vapour 32
Equation, van der Waals’ 14
Equilibrium, thermal 2
Equilibrium, thermal, conditions of 118 143 182
Equilibrium, thermal, heterogeneous 187 194
Equilibrium, thermal, of a gas mixture 223—225
Equivalent weight 23
Equivalents, number of 24
Euler 182
Eutectic mixture 264
Evaporation of ammonium carbamate 194
Evaporation of ammonium chloride 194
Exothermal process 39
Expansion, at absolute zero 277 286
Expansion, coefficient of 8
External, conditions of equilibrium 143
External, effect 41
External, in reversible process 54
External, variable 178
External, Work in complete cycle 56
Falk 260
Fanjung 245
Favre 76
First law of thermodynamics, general exposition 40—40
Free energy 113
Free energy, change of, with temperature 117
Free energy, decrease of, by dilution 116
Free energy, minimum value of 121
Free energy, of a perfect gas 117
Freezing point, depression of 200 254 291
Function 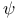 118 118
Function 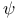 , heat (Gibbs’) 74 189 , heat (Gibbs’) 74 189
Functions, characteristic 121
Fundamental point (triple) 161
Fundamental point (triple), pressure 160
Fundamental point (triple), temperature 160
Fundamental point (triple), temperature of ice 160
Fundamental point (triple), triangle 165
Fusion, curve 162 107
Fusion, theory of 142
Gas, constant 29
Gas, energy of 216
Gas, entropy of 220—222
Gas, mixture 10
Gas, thermometer 3
Gaseous systems 215—228
Gases, perfect, ideal 5 00
Gay Lussac’s law, deviations from 130
Gay Lus’sac 25 49 60
Ghosh, J.C. 245 258 260
Gibbs 75 179 220 238
Gibbs’ phase rule 185 238
Gibbs’ phase rule, heat function 74 189
Goebel 247
Graded dissociation 227
Gram calorie, mechanical equivalent of 44
Gruncr 273
Gruneisen 277
Heat and work, analogy between 54
Heat function at constant pressure 75
Heat Theorem (Nernst’s) 272—292
Heat, absorbed 57
Heat, at constant pressure 58 126
Heat, at constant volume 58 120 277
Heat, atomic 30
Heat, capacity 35
Heat, capacity at absolute zero 276 280
Heat, latent (theory) 150
Heat, latent (theory), approximation formula 151
Heat, molecular 35
Heat, molecular, of perfect pas 61
Heat, of 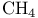 77 77
Heat, of  77 77
Heat, of combustion 77
Heat, of dilution 205
Heat, of dilution of  72 72
Heat, of dissociation 24
Heat, of formation of  70 70
Heat, of neutralization 77
Heat, of precipitation 209
Heat, of reaction 39
Heat, of reaction at absolute zero 288
Heat, of reaction at constant pressure 73
Heat, of reaction in thermochemistry 70
Heat, of solidification 209
Heat, of solution 197
Heat, of sublimation 39
Heat, of transformation of rhombic sulphur into monoclinic 279
Heat, quantity 36
Heat, specific heat, definition 35
Heat, total heat 38
Heat, unit of 34
Helmholtz 116
Henning 150 154 163
Henry’s law 249
Hertz, H. 152
Heterogeneous equilibrium 187—194
Heydweiller 240 242
Holborn 150 163
Homogeneous system 125—138
Horstmann 194
Hydriodic acid dissociation 226
Hydrobroamylene 32
Hydrogen peroxide 76
Independent constituents 179
Inertia resistance 119
Infinite dilution 72
Infinitely slow process 53
Inflect ion, point of 18
Influence of pressure on specific heat 130
Influence of temperature on combustion 78
Internal conditions of equilibrium 143
Internal conditions of equilibrium, variable 184
Internal energy 48
Internal energy of perfect gas 50
Inversion, point of 132
Iodine vapour, dissociation of 227
Irreversible diffusion 222
Isobaric change 7
Isochoric change 8
Isohydric solutions 200
Isohydric solutions, Arrhenius’ theory of 207
Isolated system 40
Isopiestic change 7
Isopynic change 8
Isostorie change 8
Isothermal isobaric process 118
Isotherms of 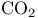 10 10
Isotropic bodies 3
Jalm 231
Joule 38
Joule and Thomson’s, absolute temperature 130
Joule and Thomson’s, experiment 49
Joule and Thomson’s, theory of, experiment 130—133
Joule’s experiment 43—44 49
Kelvin 153
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте