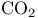|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Planck M. — Treatise on thermodynamics |
|
|
 |
| Предметный указатель |
Kirchhoff 197 205
Kirchhoffs formula for heat of saturation 205
Klebe 150 154
Knoblauch 150 154
Kohlratisch 240 242
Kundt 129
Latent energy 116
Latent heat 39 150 151
Latent heat, from phase rule 193—195
Laws of thermodynamics see “First and Second”
Laws, Avogadro’s 36 60
Laws, Babo’s 206
Laws, Boyle’s 5 60
Laws, Dalton’s 11 21
Laws, Dulong and Petit’s 36
Laws, Gay Lussac’s 6 25 49 60
Laws, Henry’s 249
Laws, Marriotte’s 5
Laws, Nernst’s 260
Laws, Neumann’s 37
Laws, Ostwald’s 245
Laws, vant’ Hoff’s 263
Laws, Wullner’s 207
Lead sulphide 71
Linde 150 154
Linde, 0von 132
Liquid air machine 132
Lowering, of freezing point 209 254 291
Lowering, of vapour pressure 207 255
Lunden 242
Mariotte’s law 5’
Massieu 121
Maximum value of 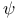 121 121
Maximum value of the entropy 120
Maximum work 114
Maxwell 90
Mechanical equivalent of a gram calorie 43
Mechanical equivalent of heat 42
Mechanical equivalent of heat in absolute units 44
Melting point of ice, lowering of, by pressure 153
Membranes, semipermeable 210
Meyer, Robert 64
Mixture of gases 10
Mixtures 21
Moating at constant pressure 58
Moating at constant volume 58
Molecular heat 36
Molecular heat of perfect gases 61
Molecular weight 23
Molecular weight, apparent 30
Molecules, number of 26
Naccari 249
Natural process, direction of 111
Nernst 240 262 269 273 280 282 288
Nernst’s law of distribution 262
Nernst’s Theorem (heat) 272—292
Neumann 37
Neutralization, heat of 75
Nitrogen, oxides 24
Nitrogen, peroxide 32
Non-variant system 185
Noyes 242 260 269
Osmotic pressure 212 256
Ostwald 70
Ostwald’s law 245
Oxides of nitrogen 24
Pagliani 249
Partial pressures 10
Passive resistance 119
Perfect gases 60
Perfect system 46
Phase, defined 179
Phase, rule 185
Phosphorus pentachloride 32
Planck 274
Planck’s quantum theory 274
Plank, R. 133
Point ( + 2)ple 185 + 2)ple 185
Point of inflection 19
Point of inversion 132
Point quadruple 186
Point quintuple 186
Point triple 101
Pollitzer, F. 282
Porous plug experiment 50
Potassium chlorate 189
Potential, energy 46
Potential, energy, thermodynamic 118
Precipitation, heat of 209
Pressure coefficient 8
Pressure of mercury 9
Pressure, fundamental 100
Pressure, fundamental, motic 212 256
Pressure, fundamental, of saturated vapour 280 283
Pressure, fundamental, reduced 20
Principle of Bertholet 110
Process, adiabatio 112
Process, endothermal 39
Process, exothermal 39
Process, isothermal 113
Process, isothermal-isobaric 118
Processes, periodic 85
Processes, reversiable and irreversible 84
Quadruple point 186
Quantity of heat 34
| Quintuple point 186
Raoult 257
Ratio of specific heats 62
Reduced pressure, temperature, volume 20
Regnault 61
Resistance, inertia 119
Reversibility of a process, condition of complete 97
Roozeboom, Bakhuis 185
Rumford 38
Saturation point 17
Schames 133
Scheel 163
Schulze 132
Second law of thermodynamics, deductions 108
Second law of thermodynamics, introduction 79
Second law of thermodynamics, possible limitations 105
Second law of thermodynamics, proof of 89
Second law of thermodynamics, test of 154
Semipermeable membranes 210
Silbermann 76
Silver acetate 250
Silver acetate, bromate 208
Silver acetate, nitrate 268
Singular values 39
Sodium carbonate 75
Sodium carbonate, hydrate 75
Solidification, heat of 209
Solutions, dilute 229—271
Solutions, isohydric 206
Solvent 204
Sound, velocity of 64
Specific density 7
Specific heat 35
Specific heat, at constant pressure 58 126
Specific heat, at constant volume 58 120
Specific heat, influence of pressure on, at constant pressure 130
Specific heat, influence of volume on, at constant volume 126
Specific heat, of saturated vapour 157
Specific heat, of steam 154
Specific heats, difference of 120
Specific heats, of mercury 127
Specific heats, ratio of specific heats of gases 62
Specificentropy 125
States of aggregation 71
States of aggregation, coexistence of 159
States, corresponding 20
Stohmann 73
Sublimation curve 162 167
Sublimation curve, theory of 142
Succinic acid 249
Sulphur 33
Sulphur, dioxide and water, equilibrium 186
Sulphur, monoclinic 279
Sulphur, rhombic 279
Sulphuric acid, dissociation of 246
Surface, developable 170
System, condensed 187
System, divariant 187
System, gaseous 215—225
System, homogeneous 125—138
System, non-variant 185
System, perfect 40
System, univariant 187
Tammann 178
Temperature, absolute 6 135—138
Temperature, critical 18 158
Temperature, critical solution 188
Temperature, definition of 2 3
Temperature, fundamental 160
Temperature, fundamental, of ice 160
Thallium chlorate 189
Theoretical regions 19
Thermal equilibrium 2
Thermochemical symbols 70
Thermodynamic potential 118
Thermodynamical theory of fusion, evaporation and sublimation 142
Thermometer, gas 3
Thiesen 147
Thomsen, J. 70 75 249
Thomson 31 90 153
Transformation, heat of rhombic sulphur to monoclinic 279
Triangle, fundamental 165
Triple point 161 180
Unit of heat 39
Univariant system 187
Van der Waals’ constants for 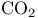 15 15
Van der Waals’ constants for 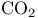 , equation 14 , equation 14
van’t Hoff 250 255
Van’t Hoff s laws 255 260 263
Vaporization, curve 162 167
Vaporization, curve, theory of 149
Vapour densities, abnormal 30
Vapour pressure, lowering of 207 255
Variable, internal and external 184
von Linde, C. 132
W.Warburg 129
Water, dissociation of 240
Weights, molecular and equivalent 23
Wiillner’s law 207
Work and heat, analogy between 54
Work and heat, external in reversible process 54
Work and heat, maximum 114
Wormann 242
Zero energy, state of 40
Zero, absolute 135—138
Zero, calorie 35
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



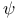
 + 2)ple
+ 2)ple