|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Parr R., Yang W. — Density-functional theory of atoms and molecules |
|
|
 |
| Предметный указатель |
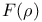 , Hohenberg — Kohn functional, bounds 60 , Hohenberg — Kohn functional, bounds 60
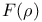 , Hohenberg — Kohn functional, constrained search definition 57 62 65 66 68 187 , Hohenberg — Kohn functional, constrained search definition 57 62 65 66 68 187
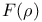 , Hohenberg — Kohn functional, definition 52 54 , Hohenberg — Kohn functional, definition 52 54
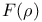 , Hohenberg — Kohn functional, differential of 89 , Hohenberg — Kohn functional, differential of 89
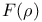 , Hohenberg — Kohn functional, Fock space 70 , Hohenberg — Kohn functional, Fock space 70
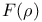 , Hohenberg — Kohn functional, multicomponent systems 215 216 , Hohenberg — Kohn functional, multicomponent systems 215 216
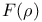 , Hohenberg — Kohn functional, scaling behavior 237—238 , Hohenberg — Kohn functional, scaling behavior 237—238
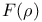 , Hohenberg — Kohn functional, spin-polarized generalization 171 , Hohenberg — Kohn functional, spin-polarized generalization 171
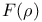 , Hohenberg — Kohn functional, subtleties in 60 , Hohenberg — Kohn functional, subtleties in 60
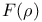 , Hohenberg — Kohn functional, universality of 53 60 63 66 68 , Hohenberg — Kohn functional, universality of 53 60 63 66 68
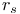 definition 272 definition 272
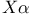 method (approximation) 154—156 281 284. method (approximation) 154—156 281 284.
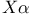 method (approximation), contrast with TFD method 156 method (approximation), contrast with TFD method 156
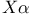 method (approximation), effective potential in 154 method (approximation), effective potential in 154
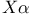 method (approximation), Hartree — Fock — Slater equation 154 method (approximation), Hartree — Fock — Slater equation 154
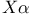 method (approximation), self-interaction corrected 183 method (approximation), self-interaction corrected 183
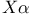 method (approximation), spin-polarized 178 method (approximation), spin-polarized 178
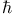 , expansion See phase-space distribution function , expansion See phase-space distribution function
Absar, I. 14 313
Absolute hardness See hardness
Acharya, P.K. 106 138 139 140 285 287 307
Acids and bases 98. See HSAB principle
Adams, W.H. 224 285
Adiabatic connection 186—189 194 203
Aicher, S. 118 305
Akai, H. 244 290
Alan, D.C. 244 308
Alder, B.J. 275 288
Allan, N.L. 136 285
Almbladh, C.-O. 152 153 285
Alonso, J.A. 138 191 233 285 286 317
Alpha parameter in 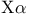 theory 154—155 183 theory 154—155 183
Alpha parameter in 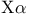 theory, recommended values for atoms 155 theory, recommended values for atoms 155
Alvarellos, J.E. 191 285
Amaldi, E. 181 292
Amaral, O.A. V. 199 285
Anderson, A. 230 308
Annihilation and creation operators 41 259—264
Antisymmetry principle 4 23 29
Arfken, G. 161 246 285
Aromaticity 98
Aryasetiawan, F. 152 285
Asbrink, L. 234 303
Ashby, N. 117 285
Ashcroft, N.W. 83 217 285 290
Atom in a molecule 90 221—224
Atom in a molecule, Bader definition 222 224
Atom in a molecule, density-functional definition 222—223
Atom in a molecule, density-matrix model 224
Atom in a molecule, in HSAB principle 225
Atom in a molecule, need for the construct 221—222
Atom in a molecule, promotion energy of 223—224
Atom in a molecule, valence state of 222 223—224
Atom in a molecule, wave-function viewpoint 222 224
Atomic charges 92 235—236
Atomic radius 95
Atoms and molecules, long-range interactions among 210. See Gordon-Kim method
Atoms, electronegativities and hardnesses of 277
Averill, F.W. 239 285
Avery, J. 281 290
Bader, R.F. W. 14 138 140 200 222 286 288 314
Baird, N.C. 93 286
Balazs, N.L. 114 115 265 286
Balbds, L.C. 138 233 286 294 317
Balduz, J.L., Jr. 70 75 224 309
Baltin, R. 138 140 244 286
Bamzai, A.S. 281
Band gap 86 96
Baroni, S. 186 212 243 286
Bartel, J. 118 305
Bartolotti, L.J. 84 85 93 124 126 127 138 139 140 149 166 167 208 212 240 285 286 287 307 308
Basis functions or set 12
Basis functions or set, continuous 22
Baskes, M.I. 243 287
Becke, A.D. 179 198 200 287 317
Bederson, B. 210 305
Berk, A. 115 135 287 308
Berkowitz, M. 102 104 119 125 227 239 241 243 287 294
Bernholc, J. 179 287
Berrios-Pagan, I. 239 314
Bertoni, C.M. 200 307
Bhaduri, R.K. 128 299
Binding energy, from charge transfer 91—92 93 225 226
Blaizot, J.-P. 35 71 77 203 259 263 287
Bloch equation 269
Blugel, S. 244 290
Boehme, R.F. 243 305
Bogdanovic, R. 183 295
Bohnen, K.-P 191 311
Boltzmann density matrix 269
Bond distance, relation to electronegativity and softness 232
Bond electronegativity 233—234
Bond hardness 233
Bond-charge model 229—234
Bond-charge model, as density-functional model 235
Bond-charge model, bond-charge values 230—231
Bond-charge model, derealization length in 230
Bond-charge model, electronegativities in 230—234
Bond-charge model, for diatomic force constants 229
Bond-charge model, hardness and softness in 232—234
Borkman, R.F. 229 231 236 287
Born — Oppenheimer approximation 3 217
Boson systems 205 216
Boyd, R.J. 95 287
Bra, ket notation 20—24
Brack, M. 118 128 204 287 299 305
Brown, J.E. 229 230 236 288 308
Brual, G., Jr. 221 288
Brueckner, K.A. 12 274 294 313
Buff, F.P. 67 313
Bylander, D.M. 86 179 302 312
Calder, G.V. 229 288
Callaway, J. 170 204 281 288 299 311
canonical ensemble 27 44 269
Canonical ensemble, Helmholtz free energy in 45 61
Canonical ensemble, partition function in 45
Canonical ensemble, zero-temperature limit 81—84
Capitani, J.F. 205 217 244 288 306 315
Car, R. 244 288 292
Carr, W.J., Jr. 274 288
Carroll, M.T. 200 288
Catalysis, local softness and 102
Cations, electronegativity and hardness of 278
Cedillo, A. 141 288
Ceperley, D.M. 275 288
Chac6n, E. 191 285
Chakrovorty, S.J. 244 293
Chandler, D. 217 288 291
Charge transfer, driven by chemical potential difference 91 224 225—226
Charge transfer, energy associated with 91
Charge-current conservation 209
Chattaraj, P.K. 138 140 288 290 297
Chayes, J.T. 54 69 288
Chayes, L. 54 69 288
Chemical binding 218 222 229. HSAB
Chemical binding in Kohn — Sham theory 218
chemical potential 70—86. See electonegativity electronegativity
Chemical potential derivatives 87—104. See hardness Fukui
Chemical potential, analogy with classical quantity 91 214 239
Chemical potential, as electronegativity 74—75 88 92 276
Chemical potential, canonical ensemble 83—84
Chemical potential, classical systems 67
Chemical potential, differential expression for 88
Chemical potential, from numerical interpolation 84 89
Chemical potential, grand canonical ensemble 45
Chemical potential, in bond-charge model 232
Chemical potential, in density-functional theory 52 142
Chemical potential, in density-matrix-functional theory 213—214
Chemical potential, in grand-canonical ensemble dft 64 66 70—74
Chemical potential, in TFDW model 113
| Chemical potential, in Thomas — Fermi theory 48 110
Chemical potential, in Thomas — Fermi — Dirac theory 109 110
Chemical potential, of atoms in a molecule 223
Chemical potential, pure state 81—83
Chemical potential, role in HSAB principle 225—228
Chemisorption 243
Chen, M.-B. 11 308
Chen, Z. 116 288
Chopra, M. 138 298
Choquet-Bruhat, Y. 252 288
Cioslowski, J. 56 60 116 163 289
Classical approximation for Wigner distribution 130 269—270
Classical systems 66 217
Clayton, M.M. 224 285
Clementi, E. 126 134 137 289
Closed-shell atoms, interaction energy 157
Closure relation 22 23
Clugston, M.J. 220 289
Cohen, J.S. 220 289
Cohen, L. 32 199 243 266 270 289
Coleman, J. 31 32 41 43 289
Colle, R. 199 289
Compton profiles 241—242
Configuration interaction 14
Connolly, J.W. D. 156 178 281 289
Constrained search 57 59 62 65 68 70 82 150 164 171 172 187 205 207 208 213 214 215 216 237
Convex (concave) functions and functionals 62 255—258
Convex (concave) functions and functionals, Jensen's inequality for 256 257
Convex set 32 43
Convex set, extreme element of 43
Cook, K. 156 289
Cooper, D.L. 136 285
Correlation (energy) 11 13—14 40 106 176 177—178 183 199
Correlation hole 190
Correlation potential 154 184—185
Correlation-energy functional 177 185 186 190
Correlation-energy functional, Colle — Salvetti formula 199
Correlation-energy functional, diatomic molecules 185
Correlation-energy functional, in Gordon — Kim method 219
Correlation-energy functional, local spin-density approximation for 178 185
Correlation-energy functional, LSD calculation of, for atoms 183 185
Correlation-energy functional, need for improved approximations for 244
Correlation-energy functional, random phase approximation 275
Correlation-energy functional, scaling behavior 239
Correlation-energy functional, uniform electron gas 177 274—275
Cortona, P. 183 289
Coulson, C.A. 97 289
Courant, R. 246 252 254 290
Covalent radius 93
Craig, D.P. 13 308
Crystal structure prediction by dft 235
Csavinsky, P. 116 290
Cummins, P.L. 140 290
Current vector 209
Curtin, W.A. 217 290
Cusp condition See electron density density first
Dahl, J.P. 281 290
Dalgarno, A. 210 290
Das, G.P. 244 290
Datta, D. 98 290
Davidson, E.R. 14 28 35 72 281 290 310
Deb, B, M. 17 138 139 140 208 212 243 244 281 282 288 290 294 297 304
Dederichs, P.H. 244 290
Delley, G. 179 290
density See electron density
Density matrix 25 281
Density matrix, and Wigner distribution function 265
Density matrix, first order 28 30.
Density matrix, first order, cusp conditions on 35
Density matrix, first order, for single determinant 35—36
Density matrix, first order, for uniform electron gas 107
Density matrix, first order, Gaussian approximation 120 122
Density matrix, first order, in terms of field operators 42
Density matrix, first order, in terms of local potential 130 159
Density matrix, first order, normalization condition 28 118
Density matrix, first order, path-integral representation 161
Density matrix, first order, spinless 32
Density matrix, for single determinant 36
Density matrix, pth-order 28 30
Density matrix, reduced 27—32
Density matrix, second-order 28 194
Density matrix, second-order, cusp conditions on 35
Density matrix, second-order, for single determinant 36 39
Density matrix, second-order, in terms of field operators 42
Density matrix, second-order, normalization condition 28
Density matrix, second-order, spinless 33
Density matrix, second-quantized representation 263—264
Density matrix, spinless 32—35
Density of states 48 108
Density of states as related to softness 102
Density operator 24—27
Density operator, and Weyl classical function 266—267
Density operator, and Wigner distribution function 265
Density operator, commutator with Hamiltonian 27
Density operator, determined by density for ground state 63 65
Density operator, ensemble 25 26 27 44 202
Density operator, first order 29 36
Density operator, first order, eigenfunctions and eigenvalues 29
Density operator, first order, idempotency condition 36—37
Density operator, first order, positive semidefinite 29
Density operator, Fock-space 45 64
Density operator, idempotent, for pure state 26
Density operator, second-order 29
Density operator, second-order, eigenfunctions and eigenvalues 29
Density operator, second-order, positive semidefinite 29
Density operator, time-dependent 27
Density-density response 210
Density-functional theory (dft) 47—69 281—284.
Density-functional theory (dft), and hydrodynamics 244
Density-functional theory (dft), and molecular dynamics 244
Density-functional theory (dft), canonical ensemble 60—64
Density-functional theory (dft), classical systems 66—69 217
Density-functional theory (dft), connection with stochastic mechanics 244
Density-functional theory (dft), for boson systems 205
Density-functional theory (dft), for constrained systems 244
Density-functional theory (dft), formulation using scaling transformations 244
Density-functional theory (dft), fundamental differential expression 87
Density-functional theory (dft), grand canonical ensemble 64—66
Density-functional theory (dft), ground state 51—60
Density-functional theory (dft), inequalities 244
Density-functional theory (dft), momentum-space 244
Density-functional theory (dft), spin-polarized 157
Density-functional theory (dft), stability conditions in 244
Density-functional theory (dft), transcription into local thermodynamics 239—243
Density-matrix-functional theory 213—215
Density-matrix-functional theory, derivation by constrained search 213
Density-matrix-functional theory, for atoms in molecules 224
Density-matrix-functional theory, in natural orbital representation 214
DePristo, A.E. 138 141 198 290 300
Dewitt-Morette, C. 252 288
Dhar, S. 179 208 291
Dhara, A.K. 209 243 291 294
Dharma-wardana, M.W. C. 204 212 291 296 310
Dillard-Bleick, M. 252 288
Ding, K. 217 291
Dinur, U. 138 312
Dipole moment 91 93
Dirac, P.A. M. 20 105 108 291
Dispersion forces 219 220
DonneUy, R.A. 74 213 214 222 291 308
Dreizler, R.M. 133 135 136 140 141 208 210 243 282 291 296 300 313
Dunlap, B.I. 179 291
Ebner, C. 66 291
Edmiston, C. 11 291
Electrochemistry 243
Electron affinity 74 166 224 243 276
Electron density 14—16 33 281
Electron density, approximation in terms of local potential 131
Electron density, as variable in semiempirical dft 234
Electron density, atomic, calculated by various methods 135
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



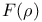 , Hohenberg — Kohn functional, bounds
, Hohenberg — Kohn functional, bounds 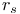 definition
definition 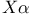 method (approximation)
method (approximation) 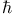 , expansion
, expansion 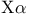 theory
theory