|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Debye P. (ed.) — The Structure of Molecules. Transl W Deans |
|
|
 |
| Предметный указатель |
"Active" vibration 28
"Diatomic" molecules 43
"Hardening" effect 33 34
"Inactive" vibration 28 32
"Promoted" electrons 168
"Rigidity" of bond 40
"Rigidity" of molecular form 54
"Triatomic" cyanogen compounds 43
"Triatomic" halogen derivatives 45
"Triatomic" molecules 44
"United atom" 157 159 161 168 173
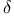 -electrons 160 -electrons 160
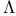 -doubling 150 156 -doubling 150 156
 -electrons 35 160 -electrons 35 160
 -vibration 42 -vibration 42
 -electrons 35 160 -electrons 35 160
 -vibration 42 -vibration 42
Absorption and dissociation 98 99 102
Absorption of benzyl chloride 11
Absorption of stilbene dichloride 8 10 11
Absorption of succinic acid derivatives 8—10
Absorption spectra 121 et seq
Absorption spectra and temperature 124 125 128 129 136 140 141
Absorption spectra continuous regions in 101 106 107 112 121
Absorption spectrum of acetaldehyde 123—124
Absorption spectrum of acetylene 30 31
Absorption spectrum of carbon disulphide 128—131
Absorption spectrum of carbon monosulpbide 129
Absorption spectrum of carbon monoxide 129
Absorption spectrum of formaldehyde 126—127
Absorption spectrum of metallic vapours 112
Absorption spectrum of methane 31
Absorption spectrum of nitrogen peroxide 25 131—134
Absorption spectrum of water vapour 31
Absorption spectrum of xenon 115
Acetaldehyde, absorption spectrum of 123—124
Acetaldehyde, predissociation of 122—124
Acetylene and nitrogen, similarity of 31 176 180
Acetylene type of molecule 47 48 49
Acetylene, absorption spectrum of 30 31
Acetylene, internuclear distances in 30
Active forms of stereo-isomers 5—12 14—17 19 20
Aldehyde-pyranose equilibrium 13 14
Alkali halid.es, dissociation of 105 106 107 108
Almasy, F. 124 137
Aluminium hydride 119 152
Ammonia type of molecule 47 50 51
Ammonia, "flat" and "pointed" molecular models of 26
Ammonia, internuclear distances in 30
Ammonia, predissociation of 127—128
Ammonia, Raman spectrum of 86
Ammonia, rotation-vibration bands of 28 29 30
Ammonia, velocity of dissociation of 128
Andrews 41 91
Angus 88
Atomic binding 107
Atomic molecules 103 109—112 116
Atomic molecules, potential curves for 110
Badger, R.M. 29 30
Band spectra see also “Absorption spectra”
Band spectra and stereochemistry 41
Band spectra of diatomic molecules 24 25 32—37
Band spectra of polyatomic molecules 23—54
Band spectra, and dissociation 97—119
Band spectra, intensity variations in 30 31 59 98 100 101 106 107 112 133 136 158
Band-head diagram 24
Bar, R. 70
Barker, E.F. 29 60 86
Bartlett, J.H. 165
Basyrin, W. 17
Bayley 88
Bengtsson 152
Bennett, W.H. 48
Benzaldehyde, predissociation of 124—126
Benzene ring 50 52 91
Benzene vapour, specific heat of 141
Benzene, predissociation of 140—141
Benzene, Raman spectrum of 91
Benzyl chloride, absorption of 11
Beryllium 170
Bewilogua, L. 33
Bhagavantam, S. 88 91 96
Binder, J.L. 30
Binding constant 36 37
Binding constant, Andrews' 41
Binding constant, of "triatomic" molecules 44
Binding constant, of carbon dioxide 53 54
Binding constant, of carbon-nitrogen-oxygen compounds 35 37
Binding constant, of chlorine derivatives of methane 39
Binding constant, of diatomic hydrides 36 37
Binding constant, of elastic rod 40
Binding constant, of hydrogen halides 37
Binding constant, of methyl halides 38
Binding constant, of polyatomic molecules 37
Binding constant, of single, double, and triple bonds 38
Binding constant, of tetrachlorides 39 53
Binding electrons 34 35 168—171 173 174 179 180
Binding electrons, in polyatomic molecules 178
Binding energy 37 116
Binding energy, of hydrogen atom in formaldehyde 127
Binding in polyatomic molecules 176—180
Binding shells 34 40
Binding, atomic 107
Binding, character of 34 35
Binding, chemical 104—116
Binding, chemical and Raman effect 83 34
Binding, harmonic 36 79
Binding, homopolar 83
Binding, ionic 107
Binding, loose 101 107 112
Binding, polar 83
Birge, R.T. 102 110 155
Bjerrum 27
Blackett, P.M.S. 102
Bodenheimer, W. 1 5 6 7 11 16 21
Bodenstein 127
Boltzmann 11
BOND see “Binding”
Bond, double 1 18 20 33 34 35 37 38 171
Bond, single 1 2 33 35 37 38
Bond, triple 30 33 35 37 38 171
Bond, valency of 167 169 171
Bonhoeffer 128 146 149
Born, M. 106 121
Boron 171
Boron hydride 179
Bragg 91
Bragg's relationship 93
Brester, C.J. 78 93 95
Briiche, E. 160 176
Budde 140
building-up principle 52 157 160
Cabannes, J. 83 84 86 89 95
Cadmium hydride 119
Cadmium vapour 113
Calcite 64 87 93—95 96
Calcite, infra-red spectrum of 94 95
Calcite, Raman spectrum of 62
Calcium hydride 152
Carbohydrates 13 14
Carbon 171
Carbon dioxide, binding constant of 53 54
Carbon dioxide, fundamental vibrations of 60
Carbon dioxide, Raman spectrum of 60 61 79 88
Carbon disulphide 61
Carbon disulphide, dissociation of 130
Carbon disulphide, predissociation of 128—131
Carbon disulphide, Raman spectrum of 61 88
Carbon monosulphide, absorption spectrum of 129
Carbon monosulphide, dissociation of 130
| Carbon monoxide 35
Carbon monoxide, absorption spectrum of 129
Carbon tetrachloride 61 82
Carbon tetrachloride, binding constant of 53
Carbon tetrachloride, Raman spectrum of 61 89 90
Carbon-nitrogen-oxygen compounds 35
Carbon-nitrogen-oxygen compounds, binding constants of 35 37
Carbon-nitrogen-oxygen compounds, internuclear distances in 33 35
Carbonyl group 35
Carboxyl group 3
Carelli, A. 65
Cario 100 101
Cassie 88
Chemical binding 104—116
Chemical binding, and Raman effect 83 84
Childs, W.H.T. 30
Chloroform, Raman spectrum of 90
Chloroform, valency vibration of 44
Christiansen 122
Christy, A. 137 138 150 173
Cis-trans isomerism 19 20
Cis-trans isomerism, of boron hydride 179
Classification of electronic terms 155—156
Combination principle 24
Combination tones 27 32 72 85
Combination tones, in Raman effect 57 58
Combination tones, selection rules for 79
Condon, E.U. 98 119 181
Convergence, limit of 102 103 104 105 127 136 148 150 151 159
Cordes, H. 102 111
Correlation of molecular terms 164—167
Coupling 79 80 146 147 156
Crystals, Raman effect in 61—64 92—95
Crystals, Raman selection rules for 93—95
Curtis, W.E. 111 137
Cyanogen compounds, "triatomic" 43
D'Or 137
Dabadghao, W.M. 90 92
Dadieu, A. 90
Darbyshire, O. 111
de Hemptinne, M. 124 137
de Lazlo, H. 121
Debye, P. 4 28 33 53
Deformation vibrations 40 41 42 49 50 52
Deformation vibrations, of "diatomic" molecules 43
Deformation vibrations, of "triatomic" molecules 44 45
Degeneracy 50 52 73 74 75 76 156 158 159 166 174 175
Degeneracy, accidental 82 88 89 90 91
Degeneracy, splitting of 82
Delbriick, M. 170
Dennison, D.M. 26 60 96
Depolarization 70 74 76 79 89 96
Diatomic hydrides, binding constants of 36 37
Diatomic hydrides, internuclear distances in 32 33
Diatomic hydrides, nuclear frequencies of 33
Diatomic molecules, band spectra of 24 25 32—37
Diatomic molecules, moment of inertia of 24 36
Diatomic molecules, nuclear frequencies of 33—35 36
Diatomic molecules, Raman effect in 83 84
Diatomic molecules, rotation bands of 27
Diatomic molecules, rotation-vibration bands of 27
Diatomic molecules, term scheme for 157
Diatomic molecules, terms of 59
Diatomic Molecules, theoretical method of obtaining term manifoldness in 157—163
Diatomic oxides, nuclear frequencies of 34
Dibromobutane 12
Dicarboxylic acids 15 17 21
Dichloro-ethane 3
Dichlorobutane 12
Dichloromethane, Raman spectrum of 90 91
Dickinson, R.G. 60 69 87 88
Dillon, R.T. 60 69
Dipole moment 2 7 55 58 63 66 97 98
Dipole moment, of aliphatic esters 3
Dipole moment, of anisole 3
Dipole moment, of phenetole 3
Dipole moment, of stereo-isomers 6
Dipole moment, of stilbene dichloride 6
Dipole moment, of substances with groups free to rotate 3
Dipole moment, of succinic acid derivatives 7—9
Dipole moment, variation with temperature 12
Dirac's theory of radiation 57
Dirac, P.A.M. 65
Dispersion formula, Kramers — Heisenberg 55.5.6 58 59 65 92
Dissociation and absorption 98 99 102
Dissociation and band spectra 97—119
Dissociation by supply of electronic energy and vibrational energy 98
Dissociation by supply of rotational energy 97 115—119 146
Dissociation by supply of vibrational energy 97 98 144 145
Dissociation, energy of 102—104 164
Dissociation, energy of oxygen 111 134 136 159 164
Dissociation, limit of 101
Dissociation, of alkali halides 105 106 107 108
Dissociation, of ammonia, velocity of 128
Dissociation, of carbon disulphide 130
Dissociation, of carbon monosulphide 130
Dissociation, of iodine monochloride 111
Dissociation, of phosgene 127
Dissociation, of sulphur dioxide 135
Dissociation, probability of 148 149 150
Dissociation, without radiation 146 147 148
Donle, H.L. 3 11
Double bond, anisotropic polarizability of 20
Double bond, binding constant of 38
Double bond, carbon 1 18 20
Double bond, in carbon-nitrogen-oxygen compounds 33 35 37
Double bond, oxygen 34 171
Double vibrations 25 43 44 49 50 51
Dunkel, M. 161
Ebert, L. 2
Eisenschitz 115
Electron affinity 37 39 105
Electron transitions in polyatomic molecules 180—181
Electronic bands 97
Electronic bands, of polyatomic molecules 24
Electronic configuration for polyatomic molecules 177
Electronic configuration method for stability 165 168 173
Electronic shells, closed 163 178
Electronic shells, succession of 161 165—167
Electronic states, even 156
Electronic states, odd 156
Electronic structure of molecules and valency 155—181
Electronic terms, classification of 155—156
Electronic terms, in polyatomic molecules 175
Electrons, binding see “Binding electrons”
Electrons, loosening see “Loosening electrons”
Energy curves 144—148
Energy of dissociation 102—104 164
Energy of oxygen 111 134 136 159 164
Energy, binding 37 116 127
Eriksson 152
Eucken 60
Even electronic states 156
Even terms 59
Even vibrations 58
Extinction coefficient, molecular 123
Eymers, J.G. 119
Farkas 128 146 149
Ferguson 136
Fermi, E. 60 61 63 64 79 93
Ferrieres 128
Fine structure 24
Finkelnburg 26
Fluorescence 24 56 62 104 133 152
Fluorine 172
Fluorite, Raman spectrum of 62 64
Forbidden lines 72 76
Formaldehyde 181
Formaldehyde and oxygen, similarity of 176
Formaldehyde type of molecule 47 48 50
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



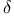 -electrons
-electrons 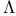 -doubling
-doubling  -electrons
-electrons  -electrons
-electrons