|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Debye P. (ed.) — The Structure of Molecules. Transl W Deans |
|
|
 |
| Предметный указатель |
Predissociation, of benzaldehyde 124—126
Predissociation, of benzene 140—141
Predissociation, of carbon disulphide 128—131
Predissociation, of formaldehyde 126—127
Predissociation, of magnesium hydride 150—152
Predissociation, of nitrogen peroxide 131—134
Predissociation, of polyatomic molecules 121—142 153
Predissociation, of sulphur 137—140 150
Predissociation, of sulphur dioxide 134—137
Pringsheim, P. 100
Probability curve for internuclear distance 99
Probability of dissociation 148 149 150
Proper frequencies of polyatomic molecules 32—54
Proper vibrations, antisymmetrical 73
Proper vibrations, degenerate 73
Proper vibrations, symmetrical 73
Pyranose forms of glucose, mannose, and galactose 13 14
Q-branch 60 69 91 132 138 150 151 152
Quantum number, rotational 69 143 156
Quantum number, vibrational 69 72 98 99 101 103 144 181
Quantum numbers of electrons in molecule 160 161 162
Quinol di-ether 3
R-branch 150 151 152
Raman effect 4 31 39 48
Raman effect, and chemical binding 83 84
Raman effect, and crystal structure 61—64 92~95
Raman effect, and molecular refraction 84
Raman effect, and molecular structure 55—61 65—96
Raman effect, and polar binding 83
Raman effect, as due to interaction between nuclear and electronic motions 65—67
Raman effect, combination tones in 57 58
Raman effect, fundamental vibrations in 58
Raman effect, in diatomic molecules 83 84
Raman effect, in hydrogen 60
Raman effect, intensity rules for 83 84 95
Raman effect, overtones in 57 58
Raman effect, rotational 58—60 87
Raman effect, selection rules for 71—82 86—91
Raman effect, vibrational 55—58
Raman scattering 57
Raman shift 59
Raman spectra 23 27 28 32 43 54
Raman spectra, intensity distribution in 59 91 92
Raman spectra, polarization in 69—71
Raman spectrum of ammonia 86
Raman spectrum of benzene 91
Raman spectrum of calcite 62
Raman spectrum of carbon dioxide 60 61 79 88
Raman spectrum of carbon disulphide 61 88
Raman spectrum of carbon tetrachloride 61 89 90
Raman spectrum of Chloroform 90
Raman spectrum of dichloromethane 90 91
Raman spectrum of fluorite 62
Raman spectrum of nitrous oxide 88
Raman spectrum of rock-salt 62 63 64
Raman spectrum of sulphur dioxide 88 89
Ramsperger, H.C 111
Rasetti, F. 55 58 60 62 63 64 69 79 88 156
Rayleigh scattering 57
Rayleigh, Lord 112
Rice, O.K. 119
Richardson 26
Ring compounds 52
Rocard, Y. 84
Rock-salt, Raman spectrum of 62 63 64
Rollefson, G.K. 103 108 111
Rosen 137 150
Rotation and vibration, interaction of 82
Rotation bands 27 59 97
Rotation bands, alternations of intensity in 30 31 59 158
Rotation bands, of diatomic molecules 27
Rotation structure 128 131 132 135 136 137 138 149
Rotation terms 59 67
Rotation-vibration bands 27 29 31 54»
Rotation-vibration bands, of ammonia 28 29 30
Rotation-vibration bands, of diatomic molecules 27
Rotational isomerism 4—6
Rotational isomers, equilibrium between 11
Rotational Raman effect 58—60 87
Rydberg 152
s-electrons 36 161
Sangewald 7
Scattering 55 56 57 65 66 67 83 84
Scattering, intensity of 68 69
Scattering, of circularly polarized radiation 70 71 90
Scattering, Raman 57
Scattering, Rayleigh 57
Sch wings 137
Schaefer, C. 50 89 94
Scheibe, G. 8 102 181
Schmidt — Ott, H.D. 107
Schonfliess 76 93
Schou, S.A. 123 126 129
Schubert 95
Schumann region 107
Schwarzschild 2S
Segre 59
Selection rules 59 95 96
Selection rules, for combination tones 79
Selection rules, for crystals 93—95
Selection rules, for infra-red spectrum 78
Selection rules, for overtones 79 85
Selection rules, for Raman effect 71—82 86—91
Selection rules, for the group 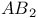 88 89 88 89
Selection rules, for the group  86 87 86 87
Shutts 91
Single bond, binding constant of 38
Single bond, carbon 1 2
Single bond, in carbon-nitrogen-oxygen compounds 33 35 37
Smekal jump 55
Snow, C.P. 88
Solvation, extramolecular 8 9 10
Solvation, intramolecular 7 8 9
Sommerfeld, A. 72
Sommermeyer, K. 106
Spectra see “Absorption spectra” “Band “Infra-red “Raman
Spin 30 150 155 156 157 163 169 171 173 175
Splitting of degeneracy 82
Splitting of Raman in carbon tetrachloride 89
Splitting of Raman line in carbon dioxide 60 61
Splitting of valencies 44 49 50 52
Sponer, H. 97 102 116 118 146 148 150 164
Stability, chemical 176 177
| Stability, electronic configuration method for 165 168 173 177
Stability, Heitler — London method for 164—165
Stability, of molecular terms 167—168
Stability, physical or molecular 176
Stemvinkel 152
Stereo-isomerism 4 5
Stereo-isomers, active forms of 5—12 14—17 19 20
Stereo-isomers, dipole moments of 6
Stereo-isomers, meso-forms of 5—12 14—17 19 20
Steric hindrance 16
Steubing, W. 112
Stilbene dichloride, absorption of 8 10 n
Stilbene dichloride, active form of 11
Stilbene dichloride, dipole moment of 6
Stilbene dichloride, meso-form of 10 11
Still 17
Stinchcomb, G.A. 29 86
Strasser, O. 11
Strong, J. 89
Stueckelberg, E.C.G. 102 168
Succinic acid active and meso-forms of 7—10
Succinic acid derivatives, absorption of 8—10
Succinic acid dipole moments of 7—9
Succinic acids, anhydrides of 14—20
Succinic acids, substituted 14—20
Sulphur dioxide, dissociation of 135
Sulphur dioxide, predissociation of 134—137
Sulphur dioxide, Raman spectrum of 88 89
Sulphur vapour and hydrogen, photo-chemical reaction between 140
Sulphur, predissociation of 137—140 150
Sun's spectrum, atmospheric lines in 31
Symmetry, ortho- or para- 156
Tamm, I. 57 93
Taylor 140
Teller, E. 82 89 92 168 169
Temperature, absorption spectra and 124 125 128 129 136 140 141
Temperature, dipole moments and 12
Terenin, A. 104
Term manifoldness 164
Term manifoldness in diatomic molecules, theoretical method of obtaining 157—163
Term manifoldness, determination from electronic configuration 159—163
Term manifoldness, determination from terms of separate atoms 157—159
Term manifoldness, determination from terms of united atom 159
Term scheme 159 161 163
Term scheme, for diatomic molecules 157
Term scheme, for polyatomic molecules 175—176
Terms of diatomic molecules 59
Terms, classification of electronic 155—156 175
Terms, correlation and stability of molecular 164—175
Terms, even 59
Terms, odd 59
Terms, rotation 59 67
Terms, vibration 67
Tetrachlorides, binding constants of 39 53
Tetrachlorides, internuclear distances in 39
Tetrachlorides, nuclear frequencies of 39
Teves 121 128 134 137 140 146 149
Tisza, L. 82
Tolansky 137
Tomaschek, R. 62
Triple bond 171
Triple bond, binding constant of 38
Triple bond, carbon 30
Triple bond, in carbon monoxide 35
Triple bond, in carbon-nitrogen-oxygen compounds 33 35 37
Triple bond, nitrogen 34
Turnbull 115
Turner 152
Valencies, angles between 26 27 33 39
Valencies, bending of 44 45 48 49 50
Valencies, splitting of 44 49 50 52
Valency and electronic structure 155—181
Valency electrons 35 37 38 83
Valency in polyatomic molecules 176—180
Valency of a bond 167 169 171
Valency theory, Heitler — London 110 116 168 169 171 173 174 177 178
Valency vibrations 40 41 42 44 48 49 52
Valency vibrations, of "diatomic" molecules 43
Valency vibrations, of "triatomic" halogen derivatives 45
van der Vleugel, E.S. 172
Van der Waals molecules 112—116
van Iddekinge, H.H. 137 149
van Leeuwen 106
van Vleck, J.H. 58 65 85 143
van Wijk, W.R. 89 96
Vibration and rotation, interaction of 82
Vibration terms 67
Vibration, types of 48
Vibrational bands, variation of intensity in 100 101 133 136
Vibrational levels 144 146
Vibrational Raman effect 55—58 60
Villars, D.S. 119
Volkert 3
von Auwers 21
von Neumann, J. 109
Walden inversion 16 21
Warburg, Ј. 127
Water type of molecule 47 48 49
Water vapour, absorption spectrum of 31
Water, "flat" and "pointed" molecular models of 26
Water, internuclear distances in 31
Watson, W. 88 128 134
Wave equation 143 144
Weissberger 7
Weizel, W. 26 109 167 170
Wierl 28
Wilson, E.D. 111 128
Winans, J.G. 102 113
Witmer, E.E. 148 157
Wolf, K.L. 1 2 3 5 6 8 11 14
Wolff, F. 136
Wood, R.W. 58 100
Wren 17
Wrigner, E. 78 109 148 157
WTest, S.S. 88
WTieland, K. 134
Wurmser 149
Xenon, absorption in 115
Yates, R.C 91
Zero-point energy 99 144
Zero-point vibration 99 101 118
Zinc hydride 119
Zinc vapour 113
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте