јвторизаци€
ѕоиск по указател€м
Carey F.A., Sundberg R.J. Ч Advanced organic chemistry (Part B)
ќбсудите книгу на научном форуме Ќашли опечатку?
Ќазвание: Advanced organic chemistry (Part B)јвторы: Carey F.A., Sundberg R.J. јннотаци€: Since its original appearance in 1977, Advanced Organic Chemistry has found wide use as a text providing broad coverage of the structure, reactivity and synthesis of organic compounds. The Fourth Edition provides updated material but continues the essential elements of the previous edition. The material in Part A is organized on the basis of fundamental structural topics such as structure, stereochemistry, conformation and aromaticity and basic mechanistic types, including nucleophilic substitution, addition reactions, carbonyl chemistry, aromatic substitution and free radical reactions. The material in Part B is organized on the basis of reaction type with emphasis on reactions of importance in laboratory synthesis. As in the earlier editions, the text contains extensive references to both the primary and review literature and provides examples of data and reactions that illustrate and document the generalizations. While the text assumes completion of an introductory course in organic chemistry, it reviews the fundamental concepts for each topic that is discussed. The Fourth Edition updates certain topics that have advanced rapidly in the decade since the Third Edition was published, including computational chemistry, structural manifestations of aromaticity, enantioselective reactions and lanthanide catalysis. The two parts stand alone, although there is considerable cross-referencing. Part A emphasizes quantitative and qualitative description of structural effects on reactivity and mechanism. Part B emphasizes the most general and useful synthetic reactions. The focus is on the core of organic chemistry, but the information provided forms the foundation for future study and research in medicinal and pharmaceutical chemistry, biological chemistry and physical properties of organic compounds.
язык: –убрика: ’ими€ /—татус предметного указател€: √отов указатель с номерами страниц ed2k: ed2k stats »здание: 4th edition√од издани€: 1938 оличество страниц: 965ƒобавлена в каталог: 16.04.2006ќперации: ѕоложить на полку |
—копировать ссылку дл€ форума | —копировать ID
ѕредметный указатель
N-hydroxysuccinimide, in activation of carboxylic acids 175Ч176 N-hydroxysuccinimide, in peptide coupling 899 N-methylpyrrolidinone as solvent 21Ч22 Nickel, organo-, compounds 525Ч529 Nickel, organo-, compounds, 526 532 Nickel, organo-, compounds, as intermediates in coupling halides and Grignard reagents 528Ч529 Nickel, organo-, compounds, coupling of halides and sulfonates by 526Ч529 Nickel, organo-, compounds, in coupling of aryl boronic acids 529 Nitration 693Ч696 Nitration, by acetyl nitrate 694 Nitration, by nitronium salts 694Ч695 Nitration, by ozone and nitrogen dioxide 695 Nitration, by trifluoroacetyl nitrate 694 Nitration, catalysis by lanthanide salts 694 Nitrenes 642Ч645 Nitrenes, alkyl, rearrangement of 644 Nitrenes, aryl, rearrangement of 644 Nitrenes, carboalkoxy 644Ч645 Nitrenes, from azides 642Ч644 Nitrenes, sulfonyl 645 Nitrenoid intermediate 616 Nitrile oxides, cycloaddition reactions 361 365 Nitriles, 489 Nitriles, 839 853 Nitriles, 556 Nitriles, alkylation 31 Nitriles, aromatic acylation by 711 Nitriles, conversion to primary amides 179 Nitriles, in epoxidation of alkenes 768 Nitriles, preparation of, by conjugate addition of cyanide 46 Nitriles, preparation of, by nucleophilic substitution 150 Nitriles, preparation of, from aryl halides 728 Nitriles, reaction with organomagnesium compounds 450 Nitriles, reduction to aldehydes 269 Nitrite esters, alkoxy radicals from 656Ч657 Nitrite esters, diazotization by 715 Nitroalkenes, as dienophiles 342 Nitroalkenes, conjugate addition reactions of 45 Nitrones, cycloaddition reactions 364Ч365 Nitrosyl chloride, as reagent 217 NMP see УN-methylpyrrolidinoneФ Normant reagents 495 Olefin metathesis, in epothilone A synthesis 893Ч894 907 Olefin metathesis, in Prelog Ч Djerassi lactone synthesis 881 Oligonucleotides, solid phase synthesis 900Ч903 Orbital symmetry requirements for, 1,3-dipolar cycloaddition 359 Orbital symmetry requirements for, Diels Ч Alder reaction 332Ч333 Orbital symmetry requirements for, [2 + 2] cycloaddition 368 Organoboron compounds see УBoranesФ Organocadmium compounds see УCadmium organo-Ф Organocerium compounds see УCerium organo-Ф Organocopper compounds see УCopper organo-Ф Organolithium compounds see УLithium organo-Ф Organomagnesium compounds see УMagnesium organo-Ф Organomercury compounds see УMercury organo-Ф Organometallic compounds with 531Ч535 Organonickel compounds see УNickel organo-Ф Organopalladium see УPalladium organo-Ф Organothallium compounds see УThallium organo-Ф Organotin compounds see УStannanesФ Organozinc compounds see УZinc organo-Ф Ortho esters, as carboxylic acid protecting groups 834 Ortho esters, in Claisen rearrangement of allylic alcohols 384 388Ч389 Ortho esters, reaction with Grignard reagents 451 Osmium tetroxide 758Ч760 786 Oxalyl chloride, in preparation of acid chlorides 116 Oxalyl chloride, in Swern oxidation 753 Oxaphosphetane, as intermediates in Wittig reaction 111Ч112 Oxazaborolidines, as catalysts for enantioselective reduction 279Ч280 Oxaziridines, sulfonyl, in oxidation of enolates 797Ч798 882 Oxazolidinones see УAcyl oxazolidinonesФ Oxazolines, alkylation of anions 38Ч39 Oxazolines, as carboxylic acid-protecting groups 837 Oxetanes, from alkene-carbonyl photocycloaddition 374Ч376 Oxime ethers, radical addition reactions 666Ч667 Oximes, Beckmann rearrangement of 650Ч651 Oxirenes, as intermediates in Wolff rearrangement 641Ч642 Oxonium ylides 637Ч639 Oxy-Cope rearrangement 382Ч383 Oxy-Cope rearrangement, anionic 382 Oxy-Cope rearrangement, in synthesis of juvabione 853Ч854 Oxygen, reaction with, enolates 800Ч802 Oxygen, reaction with, radical intermediates 198 Oxygen, singlet, generation of 782 Oxygen, singlet, lifetime of 782 Oxygen, singlet, reaction with alkenes 782Ч786 Oxymercuration 196Ч200 Oxymercuration, stereoselectivity of 200 Ozonolysis 788Ч790 Palladium, organo- compounds, 499Ч500 Palladium, organo- compounds, 501Ч503 Palladium, organo- compounds, as reaction intermediates in 499Ч525 Palladium, organo- compounds, as reaction intermediates in, conversion of alkenyl halides to esters by carbonylation 521Ч525 Palladium, organo- compounds, as reaction intermediates in, coupling of alkynes and alkenyl halides 510 Palladium, organo- compounds, as reaction intermediates in, coupling of halides and organometallic reagents 507Ч510 Palladium, organo- compounds, as reaction intermediates in, nucleophilic aromatic substitution 730Ч731 Palladium, organo- compounds, as reaction intermediates in, oxidation of alkenes 501 Palladium, organo- compounds, as reaction intermediates in, reaction of aryl halides and alkenes 503Ч507 Palladium, organo- compounds, catalysis of cleavage of allylic carbamates and carbonates 830Ч832 Palladium, organo- compounds, catalysts for nucleophilic aromatic substitution 730Ч731 Palladium, organo- compounds, formation by oxidative addition 499 504 522 Paterno Ч Buchi reaction 374 Pericyclic reactions, definition 331 Periodate ion, cleavage of diols 786 790Ч791 Permanganate ion, oxidation of, alkenes 757Ч758 Permanganate ion, oxidation of, alkynes 758 Permanganate ion, oxidation of, aromatic side-chains 807 Peroxycarboxylic acids, epoxidation of alkenes 767Ч772 Peroxycarboxylic acids, oxidation of ketones 798Ч801 Peterson reaction 120Ч121 Phase transfer catalysis 149Ч150 505 623 Phenolate anions, C- versus O-alkylation of 27Ч28 Phenylselenenyl halides, as reagents 213Ч215 Phenylselenenyl sulfate, as reagent 214 Phosphate esters, alkenyl, reduction of 296 Phosphate esters, aryl, reduction of 296 Phosphines, as catalysts for O-acylation 168 Phosphines, as ligands in palladium catalysts 508 730 Phosphines, chiral, in hydrogenation catalysts 255Ч259 Phosphite esters, dialkyl, as hydrogen atom donors 290 Phosphite esters, preparation under Mitsunobu conditions 154Ч155 Phosphonate carbanions, Wittig reactions of 116Ч117 Phosphonate esters, preparation of 158Ч159 Phosphonium salts, alkoxy, as intermediates in nucleophilic substitution 144Ч145 Phosphonium salts, cyclopropyl, as synthetic equivalent groups 842Ч844 Phosphonium salts, deprotonation of 111 Phosphonium salts, preparation of 112 Phosphonium salts, vinyl, as dienophiles 343 Phosphoramidate method for nucleotide coupling 901 Phosphorus tribromide, in preparation of alkyl bromides 143Ч144 Phosphorus ylides 111Ч112 Phthalimide, as amine-protecting group 833 Phthalimide, in synthesis of amines 155Ч156 Pinacol borane, hydroboration by 229 Pinacol rearrangement 602Ч607 Pinacol rearrangement, in synthesis of longifolene 861Ч862 pK values for carbon acids 4 Polyene cyclization 598Ч602 Polypeptides, solid phase synthesis 987Ч900 Potassium ferrate 751 Prelog Ч Djerassi lactone, stereoselective synthesis of 869Ч881 Protective groups for 822Ч838 Protective groups for, amides, 2,4-dimethoxyphenyl 832 Protective groups for, amides, 4-methoxyphenyl 832 Protective groups for, amines 831Ч835 Protective groups for, amines, 832 Protective groups for, amines, allyloxycarbonyl 831Ч832 Protective groups for, amines, amides as 834 Protective groups for, amines, carbobenzyloxy 831 Protective groups for, amines, o-nitrobenzyloxycarbonyl 832 Protective groups for, amines, phthalimides as 833 Protective groups for, amines, silyl derivatives 834 Protective groups for, amines, sulfonamides as 834 Protective groups for, amines, t-butoxycarbonyl 831 Protective groups for, amines, trifluoroacetyl 833 Protective groups for, carbonyl compounds 835Ч837 Protective groups for, carbonyl compounds, acetals 835 Protective groups for, carbonyl compounds, dioxolanes 835Ч836 Protective groups for, carbonyl compounds, dithioketals 836Ч837 Protective groups for, carbonyl compounds, oxathiolanes 836 Protective groups for, carboxylic acids 837Ч838 Protective groups for, carboxylic acids, 837 Protective groups for, carboxylic acids, ortho esters 838 Protective groups for, carboxylic acids, oxazolines 837 Protective groups for, carboxylic acids, t-butyl esters 837 Protective groups for, hydroxyl groups 822Ч831 Protective groups for, hydroxyl groups, 824 Protective groups for, hydroxyl groups, 1-ethoxyethyl 823 Protective groups for, hydroxyl groups, 3,5-dimethoxybenzyl 826 Protective groups for, hydroxyl groups, 4,4'-dimethoxytriphenylmethyl 900 Protective groups for, hydroxyl groups, 4-methoxybenzyl 826 Protective groups for, hydroxyl groups, allyl 827 Protective groups for, hydroxyl groups, allyoxycarbonyl 830 Protective groups for, hydroxyl groups, benzyl 825Ч827 Protective groups for, hydroxyl groups, cyanoethyl 901 Protective groups for, hydroxyl groups, methoxymethyl 824 Protective groups for, hydroxyl groups, methoxyphenyl 827 Protective groups for, hydroxyl groups, methylthiomethyl 824Ч825 Protective groups for, hydroxyl groups, silyl ethers 827Ч829 Protective groups for, hydroxyl groups, t-butyl 825 Protective groups for, hydroxyl groups, tetrahydropyranyl 823 Protective groups for, hydroxyl groups, trichloroethyl carbonate esters 825 Protective groups for, hydroxyl groups, triphenylmethyl 825 Pummerer reaction 824 Pyrazolines, conversion to cyclopropanes 362 406 Pyrazolines, from dipolar cycloaddition reactions 360Ч361 866Ч867 Pyridazines, Diels Ч Alder reactions of 407 Pyridine-2-thiol esters as acylating agents 170Ч171 Pyridine-2-thione, N-hydroxy esters, radicals from 653 675 Pyridines, 2-halo, nucleophilic substitution reactions of 724 Pyridines, as catalysts for acylation 166 Pyridinium chlorochromate 750 Pyridinium dichromate 750 Pyrones, Diels Ч Alder addition reactions of 348 868 883 Quinodimethanes, as Diels Ч Alder dienes 345Ч347 Quinodimethanes, from benzo[b]thiophene dioxides 404 Quinones, as dienophiles 339 Radicals, 5-hexenyl, cyclization of 198 283 435 Radicals, alkoxy 656 674 678Ч679 Radicals, aryl, from N-nitrosoacetanilides 733 Radicals, aryl, reactions of 731Ч734 Radicals, as intermediates 651Ч652 654Ч679 Radicals, as intermediates, in preparation of organomagnesium compounds 435 Radicals, cyclization of 198 283 435 660Ч674 Radicals, cyclization of, regioselectivity and stereoselectivity in 660Ч662 665 Radicals, fragmentation reactions of 674Ч679 Radicals, generation of 652Ч654 Radicals, generation of, by Mn(III) oxidation 551 Radicals, generation of, by reduction of organomercury compounds 196Ч198 654 659 Radicals, generation of, from halides 652Ч654 Radicals, generation of, from N-hydroxypyridine-2-thione esters 652Ч653 Radicals, generation of, from selenides 653 666 Radicals, generation of, from thiono esters 290 665 Radicals, in aromatic substitution 731Ч734 Radicals, intramolecular hydrogen abstraction by 654Ч657 Radicals, rearrangement reactions of 674Ч679 Radicals, substituent effects on reactivity 657Ч658 Radicals, trapping of, by alkenes 657 660Ч667 Radicals, trapping of, by oxygen 198 Ramberg Ч Baecklund rerrangement 611 Red-Al see УSodium bis(2-methoxyethoxy)aluminum hydrideФ Reduction, by hydride donors 262Ч273 Reduction, by hydride donors, stereoselectivity of 273Ч277 Reduction, dissolving metal 290Ч295 Reductive amination 269Ч270 Resolution, in enantioselective synthesis 847 Retrosynthetic analysis 845Ч846 Rhodium compounds, as catalyst for, carbenoid addition and insertion reactions 632Ч637 Rhodium compounds, as catalyst for, decarbonylation 431 Rhodium compounds, as catalyst for, Fischer Ч Tropsch process 530 Rhodium compounds, as catalyst for, homogeneous hydrogenation 253 Rhodium compounds, as catalyst for, hydroboration 229Ч230 232 Rhodium compounds, as catalyst for, hydroformylation 529Ч530 Rhodium compounds, as catalyst for, hydrosilation 567 Rhodium compounds, as catalyst for, isomerization of organoboranes 232 Rink linker in oligonucleotide synthesis 899 Robinson annulation reaction 89Ч95 Ruthenium catalysts for hydrogenation 255Ч256 Samarium diiodide, reduction by 298 304Ч305 887 Sandmeyer reaction 717 Schiff base see УIminesФ Schmidt reaction 649 Selectrides see УTrialkylborohydridesФ Selenenyl halides, addition reactions with alkenes 213Ч216 806 Selenides, preparation of 213Ч216 410 Selenides, preparation of, 806 Selenides, preparation of, 781 Selenium dioxide 802 805Ч806 Selenocyclization 213Ч214 Selenoxides, allylic, [2,3]-sigmatropic rearrangements of 395 806 Selenoxides, in conversion of alkenes to allylic alcohols 806Ч807 Selenoxides, in conversion of epoxides to allylic alcohols 781 Selenoxides, preparation from selenides 410 Selenoxides, thermal elimination reactions of 410 Shapiro reaction 309Ч310 444 Sharpless asymmetric epoxidation 762Ч764 Sharpless asymmetric epoxidation, in synthesis of Prelog Ч Djerassi lactone 878Ч880 Silanes, alkenyl, epoxidation and conversion to ketones 780 Silanes, alkenyl, reactions with electrophiles 567Ч568 596 Silanes, allylic, 120Ч121 Silanes, allylic, arylation by Heck reaction 505Ч507 Silanes, allylic, as hydride donors 286Ч287 Silanes, allylic, as hydrogen atom donors 290 314 658 664 Silanes, allylic, carbanions of 120Ч121 Silanes, allylic, in polyene cyclizations 600Ч601 Silanes, allylic, reaction with electrophiles 567Ч570 596 Silanes, allylic, reactions with 575Ч576 Silanes, allylic, synthesis of 563Ч567 Silyl enol ethers, aldol addition reactions of 78Ч82 Silyl enol ethers, alkylation of 596Ч597 Silyl enol ethers, conjugate addition of 41 45 Silyl enol ethers, conversion to 779Ч780 797 Silyl enol ethers, enolates from 11 Silyl enol ethers, epoxidation and rearrangement of 780 797 Silyl enol ethers, halogenation of 219 Silyl enol ethers, in Mukaiyama reactions 78Ч82 Silyl enol ethers, of ester enolates, Claisen rearrangement of 389Ч390 874Ч876 Silyl enol ethers, of ester enolates, stereoselective formation 389 Silyl enol ethers, oxidation of 796Ч797 Silyl enol ethers, photochemical cycloaddition 376 Silyl enol ethers, preparation from trimethylsilyl esters and Lombardo's reagent 463 Silyl ketene acetals, Claisen rearrangement of 389Ч390 Simmons Ч Smith reagent 626 Sodium bis-(2-methoxyethoxy)aluminum hydride 266 268 Sodium borohydride 264Ч265 Sodium cyanoborohydride 266 269 307 Solid phase synthesis 897Ч903 Solvent effects, in enolate alkylation 20Ч23 Solvent effects, in nucleophilic substitution 147Ч150 Solvent effects, on Diels Ч Alder reactions 339 Solvents, polar aprotic 21 Stannaries, 444 578Ч579 Stannaries, 444 Stannaries, 578 Stannaries, alkenyl, palladium-catalyzed coupling with alkenyl trifluoromethanesulfonates 515 Stannaries, alkenyl, palladium-catalyzed coupling with halides 511Ч515 Stannaries, alkenyl, reactions with carbocations 596 Stannaries, allylic, radical substitution reactions of 660 Stannaries, allylic, reactions with acetals 583 Stannaries, allylic, reactions with aldehydes 579Ч582
–еклама
 |
|
ќ проекте
|
|
ќ проекте



 -allyl complexes
-allyl complexes  -unsaturated, addition of organocopper reagents to
-unsaturated, addition of organocopper reagents to  -alkoxy, as acyl anion equivalents
-alkoxy, as acyl anion equivalents 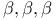 -trichloroethyloxycarbonyl
-trichloroethyloxycarbonyl -methoxyethoxymethyl
-methoxyethoxymethyl  -unsaturated carbonyl compounds
-unsaturated carbonyl compounds