јвторизаци€
ѕоиск по указател€м
Carey F.A., Sundberg R.J. Ч Advanced organic chemistry (Part B)
ќбсудите книгу на научном форуме Ќашли опечатку?
Ќазвание: Advanced organic chemistry (Part B)јвторы: Carey F.A., Sundberg R.J. јннотаци€: Since its original appearance in 1977, Advanced Organic Chemistry has found wide use as a text providing broad coverage of the structure, reactivity and synthesis of organic compounds. The Fourth Edition provides updated material but continues the essential elements of the previous edition. The material in Part A is organized on the basis of fundamental structural topics such as structure, stereochemistry, conformation and aromaticity and basic mechanistic types, including nucleophilic substitution, addition reactions, carbonyl chemistry, aromatic substitution and free radical reactions. The material in Part B is organized on the basis of reaction type with emphasis on reactions of importance in laboratory synthesis. As in the earlier editions, the text contains extensive references to both the primary and review literature and provides examples of data and reactions that illustrate and document the generalizations. While the text assumes completion of an introductory course in organic chemistry, it reviews the fundamental concepts for each topic that is discussed. The Fourth Edition updates certain topics that have advanced rapidly in the decade since the Third Edition was published, including computational chemistry, structural manifestations of aromaticity, enantioselective reactions and lanthanide catalysis. The two parts stand alone, although there is considerable cross-referencing. Part A emphasizes quantitative and qualitative description of structural effects on reactivity and mechanism. Part B emphasizes the most general and useful synthetic reactions. The focus is on the core of organic chemistry, but the information provided forms the foundation for future study and research in medicinal and pharmaceutical chemistry, biological chemistry and physical properties of organic compounds.
язык: –убрика: ’ими€ /—татус предметного указател€: √отов указатель с номерами страниц ed2k: ed2k stats »здание: 4th edition√од издани€: 1938 оличество страниц: 965ƒобавлена в каталог: 16.04.2006ќперации: ѕоложить на полку |
—копировать ссылку дл€ форума | —копировать ID
ѕредметный указатель
Diazo compounds, reactions with ketones 608Ч609 Diazoketones see УKetones diazoФ Diazomethane, in preparation of methyl esters 152Ч153 Diazomethane, in ring expansion of ketones 608Ч609 Diazonium ions, aromatic 714Ч722 Diazonium ions, aromatic, conversion to aryl azides 721 Diazonium ions, aromatic, conversion to aryl halides 719Ч721 Diazonium ions, aromatic, phenols from 717 Diazonium ions, aromatic, preparation of 714Ч715 Diazonium ions, aromatic, radicals from 731 Diazonium ions, aromatic, reaction with thiolates 715 721 Diazonium ions, aromatic, reductive dediazonation of 716Ч717 Diazonium ions, as intermediates in ketone ring expansion 608 Diborane, addition to alkenes 226Ч228 Diborane, as reducing agent 267 270Ч271 Diborane, as reducing agent, amides 270Ч271 Diborane, as reducing agent, carboxylic acids 270 Diborane, as reducing agent, epoxides 778 Dicyanodichloroquinone, oxidative removal of protecting groups 826 Dicyclohexylcarbodiimide, activation of carboxylic acids by 169 172Ч177 830 899 Dieckmann condensation 103 Diels Ч Alder reaction 332Ч359 Diels Ч Alder reaction, asymmetric 349Ч353 Diels Ч Alder reaction, catalysis by Lewis acids 336Ч338 349 Diels Ч Alder reaction, enantioselective 353Ч353 357 Diels Ч Alder reaction, frontier orbitals in 332Ч335 Diels Ч Alder reaction, intramolecular 353Ч359 404 883 Diels Ч Alder reaction, inverse electron demand 333 407 Diels Ч Alder reaction, of cyclopentadienones 405 Diels Ч Alder reaction, of pyridazines 407 Diels Ч Alder reaction, of pyrones 348 883 885 Diels Ч Alder reaction, of quinodimethanes 345Ч347 Diels Ч Alder reaction, of triazines 407 Diels Ч Alder reaction, regioselectivity of 333Ч334 Diels Ч Alder reaction, stereoselectivity 334 Diels Ч Alder reaction, transition state of 335 Dienes, Diels Ч Alder reactions of 345Ч348 Dienes, intramolecular photocycloaddition of 369 Dienes, preparation from, 2,5-dihydrothiophene-l,l-dioxides 404 Dienes, preparation from, alkenyl halides and alkenyl boranes 520 Dienes, preparation from, alkenyl halides and alkenyl Grignard reagents 508 Dienes, preparation from, alkenyl halides by nickel-catalyzed coupling 527 Dienes, reaction with singlet oxygen 786 Dienophiles 339Ч344 Dienophiles, as synthetic equivalent groups 340Ч343 Dienophiles, benzyne as 727 Dienophiles, masked functionality in 340Ч343 Diethylaluminum cyanide 46 Diimide, generation and reduction by 262 Diisobutylaluminum hydride for reduction of, amides 269 Diisobutylaluminum hydride for reduction of, esters and lactones 268 Diisobutylaluminum hydride for reduction of, nitrites 269 Diisobutylaluminum hydride for reduction of, unsaturated ketones 272 Dimethyl sulfide, chlorination in oxidation of alcohols 754Ч755 Dimethyl sulfoxide, as polar aprotic solvent 3 21Ч22 27 149 203 280 408 Dimethyl sulfoxide, in conversion of alkenes to bromohydrins 203 Dimethyl sulfoxide, in oxidation of alcohols 752Ч755 Dimethyl sulfoxide, in Pummerer reaction 824 Dimethylboron bromide, cleavage of ethers by 159Ч163 Dimethylformamide, as hydrogen atom donor 716 Dimethylformamide, as solvent 21Ч22 27 149 280 728 Dimethylpropyleneurea, solvation by 389 438 Dimethylsulfonium methylide 122Ч126 Dimethylsulfoxonium methylide 122Ч126 Dioxanes, alkenyl as dienophiles 350 Dioxetanes 784Ч785 Dioxiranes, as oxidants 771Ч772 893 Dioxolanes, alkenyl, as dienophiles 343Ч344 350 Dioxolanes, as carbonyl-protecting groups 835Ч836 Dioxolanes, reactions with allylic silanes 572Ч573 Diphenylphosphoryl azide, activation of carboxylic acids by 176Ч177 Dipolar cycloaddition reactions 359Ч367 Dipolar cycloaddition reactions, enantioselective 365 Dipolar cycloaddition reactions, intramolecular 364Ч365 Dipolar cycloaddition reactions, regioselectivity in 360Ч361 Dipolar cycloaddition reactions, stereoselectivity in 360Ч361 Dipolarophiles 359 Dithianes, as acyl anion equivalent 840 856 Dithianes, lithiation of 840 Dithioketals, as carbonyl protecting groups 836Ч838 862 DMF see УDirnethylformamideФ DMPU see УDimethylpropyleneureaФ DMSO see УDimethyl sulfoxideФ Double stereodifferentiation 83Ч84 872 Electrophilic aromatic substitution 693Ч714 Elimination reactions, carbenes from 623Ч625 Elimination reactions, cheletropic 403Ч404 Elimination reactions, of 120Ч121 Elimination reactions, of diazenes 403 Elimination reactions, thermal 408Ч414 Enammes, alkylation of 1 31Ч34 Enammes, conjugate addition to enones 46Ч47 Enammes, cycloaddition with nitrogen heterocycles 407 Enammes, halogenation of 219 Enammes, nucleophilicity 32 Enammes, of cyclohexanone 33 Enammes, photochemical cycloaddition 376 Enammes, preparation of 31Ч32 Enammes, [2 + 2] cycloaddition reactions of 370 Enantioselective catalysts in, aldol addition reactions 88Ч90 Enantioselective catalysts in, alkylzinc addition to aldehydes 461 Enantioselective catalysts in, allylic oxidation of alkenes 804 Enantioselective catalysts in, carbene insertion reactions 637 Enantioselective catalysts in, conjugate addition reactions of organometallic reagents to enones 494 Enantioselective catalysts in, Diels Ч Alder reactions 350Ч355 Enantioselective catalysts in, dihydroxylation of alkenes 759Ч760 Enantioselective catalysts in, dipolar cycloaddition reactions 365 Enantioselective catalysts in, ene reactions 401 Enantioselective catalysts in, epoxidation 762Ч766 772 Enantioselective catalysts in, palladium-catalyzed alkylation of malonate esters 502 Enantioselective catalysts in, Robinson annulation 95 Enantioselective reactions, addition of allylic boranes to aldehydes 561Ч563 Enantioselective reactions, addition of organocopper reagents to enones 491Ч494 Enantioselective reactions, addition of organozinc reagents to aldehydes 461 Enantioselective reactions, aldol additions of N-acyl oxazolidinones 85Ч87 Enantioselective reactions, aldol condensations involving double stereodifferentiation 83Ч89 Enantioselective reactions, alkylation of alkynes by organoboranes 556Ч559 Enantioselective reactions, alkylation of hydrazones 36Ч37 Enantioselective reactions, alkylation of N-acyl oxazolidinones 30Ч31 Enantioselective reactions, alkylation of oxazolines 38Ч39 Enantioselective reactions, Cope rearrangement of 1,5-dienes 379Ч380 Enantioselective reactions, Diels Ч Alder additions 349Ч353 Enantioselective reactions, epoxidation of allylic alcohols 762Ч764 Enantioselective reactions, hydroboration 230 236Ч238 Enantioselective reactions, hydrogenation 253Ч259 Enantioselective reactions, in synthesis of longifolene 876 Enantioselective reactions, ketones from organoboranes 553Ч554 Enantioselective reactions, Robinson annulation reaction 95 Ene reaction 399Ч403 Enol ethers, Diels Ч Alder reactions with nitrogen heterocycles 408 Enol ethers, lithiation of 840 Enol ethers, of 273 Enol ethers, oxidation by lead tetraacetate 796 Enol ethers, oxidation by singlet oxygen 784Ч785 Enol ethers, photochemical cycloaddition 376 Enol ethers, preparation from esters by Lombardo's reagent 462 Enol ethers, regioselectivity in Heck reaction 505 Enol phosphate esters, nickel-catalyzed coupling with Grignard reagents 529 Enol phosphate esters, reduction of 296 Enol silyl ethers see УSilyl enol ethersФ Enol sulfonate esters 309 515 885 Enolates, acylation of 101Ч110 Enolates, alkylation of 1 11Ч20 Enolates, alkylation of, by conjugate addition 39Ч44 Enolates, alkylation of, intramolecular 26 Enolates, alkylation of, O- versus C- 23Ч28 Enolates, arylation by palladium catalyzed substitution 510 Enolates, boron, aldol addition reactions of 71Ч74 86Ч88 Enolates, boron, formation from ketones 71Ч72 Enolates, carboxylation of 108 Enolates, formation of 1Ч11 Enolates, formation of, by reduction of 11 292Ч293 Enolates, formation of, enantioselective 8Ч9 Enolates, formation of, from 9Ч10 Enolates, formation of, from enol acetates 10 Enolates, formation of, from trimethylsilyl enol ethers 10Ч11 Enolates, formation of, in competition with Grignard addition 447 Enolates, formation of, kinetic versus thermodynamic control of 5Ч8 Enolates, formation of, regioselectivity of 5Ч8 Enolates, formation of, stereoselectivity of 8Ч9 65Ч66 Enolates, halogenation of 219 Enolates, in aromatic 734Ч735 Enolates, in Robinson annulation reaction 89Ч95 Enolates, magnesium, acylation of 105Ч108 Enolates, of 27 Enolates, of 27 Enolates, of cyclohexanones, stereoselective alkylation 17Ч18 Enolates, of decalones, stereoselective alkylation 18Ч19 Enolates, of esters, acylation of 101Ч108 Enolates, of esters, stereoselective formation 389 Enolates, of isopropyl phenyl ketone, O- versus C-alkylation 25 Enolates, of N-acyl oxazolidinones, aldol addition reactions 75 85Ч86 Enolates, of N-acyl oxazolidinones, enantioselective alkylation 30Ч31 Enolates, oxidation by, 798 Enolates, oxidation by, molecular oxygen 800Ч802 Enolates, oxidation by, sulfonyloxaziridines 797Ч798 Enolates, reactions with, benzene-chromium tricarbonyl complex 534Ч535 Enolates, reactivity, effect of, counter ion 23 Enolates, reactivity, effect of, crown ethers 23 Enolates, reactivity, effect of, hexamethylphoshoric triamide 23 Enolates, reactivity, effect of, solvents 21Ч23 Enolates, reactivity, effect of, tetramethylethylenediamine 23 Enolates, selenenylation of 220 Enolates, structures of 24 Enolates, sulfenylation of 220 Enolates, tin 76Ч77 89 Enolates, titanium 74Ч75 Enolates, zirconium 77 Enols, in aldol condensations 57Ч60 Enols, in halogenation of ketones 216Ч220 Epothilone A, synthesis 890Ч896 Epoxides, preparation from, 127 Epoxides, preparation from, alkenes by epoxidation 767Ч772 Epoxides, preparation from, allylic alcohols by epoxidation 762Ч764 772 Epoxides, preparation from, amines 775 903 Epoxides, preparation from, azide ion 776 Epoxides, preparation from, carbonyl compounds and sulfur ylides 125Ч126 Epoxides, preparation from, cyanide ion 776 Epoxides, preparation from, diethylaluminum cyanide 776 Epoxides, preparation from, hydrogen bromide 775 Epoxides, preparation from, organocopper reagents 487 Epoxides, preparation from, organolithium compounds 454 Epoxides, preparation from, selenide ions 781 Epoxides, rearrangement to carbonyl compounds 778Ч779 Epoxides, reduction to alcohols 284 776Ч778 878 Epoxides, ring-opening reactions 772Ч778 Epoxides, trimethylsilyl, preparation of 127 Esters, 68Ч70 Esters, 389Ч391 Esters, 391 Esters, 556 Esters, 636Ч637 Esters, 489 Esters, 494Ч495 Esters, 521 Esters, 576 Esters, 272 Esters, 11Ч14 23Ч25 41 Esters, 501 Esters, 101Ч109 Esters, 502Ч503 Esters, acetylenic, addition of organocopper reagents to 493 Esters, as alcohol-protecting groups 829Ч831 Esters, condensation reactions of 101Ч105 Esters, conversion to, amides 177 Esters, conversion to, enol ethers by Lombardo's reagent 462 Esters, enantioselective synthesis of 30Ч31 Esters, enolates of, acylation 101Ч108 Esters, enolates of, aldol addition reactions of 68Ч70 Esters, enolates of, alkylation of 28 Esters, enolates of, chelation of 69Ч70 Esters, enolates of, stereoselective formation 68 389Ч390 Esters, formate, formation of hydroxymethylene derivatives by 108Ч109 Esters, organozinc derivatives of 462 Esters, preparation, by acylation of alcohols 172 Esters, preparation, by alkylation of carboxylate ions 152Ч154 Esters, preparation, by Favorskii rearrangement 609Ч611 Esters, preparation, by Fischer esterification 172 Esters, preparation, by palladium-catalyzed carbonylation 521Ч525 Esters, preparation, from aldehydes by oxidation 795Ч796 Esters, preparation, from carboxylate salts by alkylation 153 Esters, preparation, from carboxylic acids by oxidative decarboxylation 792Ч793 Esters, preparation, from carboxylic acids using diazomethane 152Ч153 Esters, preparation, from ketones by Baeyer Ч Villiger oxidation 798Ч800 Esters, preparation, from organoboranes and 555Ч556 Esters, preparation, from ozonides 789 Esters, pyrolysis of 410Ч413 Esters, pyrolysis of, in synthesis of longifolene 868 Esters, reaction with organomagnesium compounds 446Ч447 Esters, reduction, by calcium borohydride 266 Esters, reduction, by lithium aluminum hydride 265 Esters, reduction, by lithium borohydride 266 Esters, thermal elimination 410Ч413 Esters, xanthate, pyrolysis of 413 Ethers, 789 Ethers, alkenyl see УEnol ethersФ Ethers, allyl phenyl, Claisen rearrangement of 394 Ethers, allyl vinyl, Claisen rearrangement of 383Ч384 Ethers, cleavage of 159Ч161 Ethers, preparation by nucleophilic substitution 152 Favorskii rearrangement 609Ч611 Ferrocence 533 Fischer esterification 172 Fischer Ч Tropsch process 530 Fluoride ion, as catalyst for conjugate addition 41 Fluoride ion, in reactions of allylic silanes 573Ч574 576 Fluorination, aromatic 698 Fluorine, addition to alkenes 204Ч205 Formylation, aromatic 710Ч711 Fragmentation reactions 315 612Ч614 651 793Ч794 Fragmentation reactions, radical 674Ч679 Free radicals see УRadicalsФ Friedel Ч Crafts acylation reactions 704Ч711 Friedel Ч Crafts acylation reactions, intramolecular 707 Friedel Ч Crafts acylation reactions, regioselectivity of 706 Friedel Ч Crafts alkylation reactions 699Ч704 Friedel Ч Crafts alkylation reactions, catalysts for 703 Friedel Ч Crafts alkylation reactions, chloromethylation 710 Friedel Ч Crafts alkylation reactions, intramolecular 704 Friedel Ч Crafts alkylation reactions, rearrangement during 702 704 Frontier orbitals of, 1,3-dipolarcycloadditions 360Ч362 366 Frontier orbitals of, Diels Ч Alder reactions 332Ч335 Frontier orbitals of, ene reactions 400 Frontier orbitals of, ketene cycloaddition reactions 368 Frontier orbitals of, radical reactions 657 Gif oxidation 809 Glycols see У1 Grignard reagents see УMagnesium organo- Grob fragmentation 315 612Ч614 Halides, alkenyl, from alkenyl boranes 239Ч240 Halides, alkenyl, nickel-catalyzed coupling of 527 Halides, alkenyl, palladium-catalyzed coupling with alkenyl boranes 520Ч521 Halides, alkenyl, palladium-catalyzed reaction with alkenyl stannanes 511Ч515 Halides, alkenyl, palladium-catalyzed reaction with Grignard reagents 507Ч510 Halides, alkenyl, palladium-catalyzed reaction with organolithium compounds 507Ч510 Halides, alkenyl, palladium-catalyzed reaction with organozinc compounds 508 Halides, alkyl, by addition of hydrogen halides to alkenes 191Ч196 Halides, alkyl, enantioselective synthesis of 238 Halides, alkyl, preparation from alcohols 142Ч147 Halides, alkyl, reductive dehalogenation 288Ч290 296
–еклама
 |
|
ќ проекте
|
|
ќ проекте



 -
-  -hydroxysilanes
-hydroxysilanes  -enones
-enones 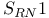 substitution reactions
substitution reactions  -pyridine-HMPA
-pyridine-HMPA  -allylpalladium compounds
-allylpalladium compounds