јвторизаци€
ѕоиск по указател€м
Carey F.A., Sundberg R.J. Ч Advanced organic chemistry (Part B)
ќбсудите книгу на научном форуме Ќашли опечатку?
Ќазвание: Advanced organic chemistry (Part B)јвторы: Carey F.A., Sundberg R.J. јннотаци€: Since its original appearance in 1977, Advanced Organic Chemistry has found wide use as a text providing broad coverage of the structure, reactivity and synthesis of organic compounds. The Fourth Edition provides updated material but continues the essential elements of the previous edition. The material in Part A is organized on the basis of fundamental structural topics such as structure, stereochemistry, conformation and aromaticity and basic mechanistic types, including nucleophilic substitution, addition reactions, carbonyl chemistry, aromatic substitution and free radical reactions. The material in Part B is organized on the basis of reaction type with emphasis on reactions of importance in laboratory synthesis. As in the earlier editions, the text contains extensive references to both the primary and review literature and provides examples of data and reactions that illustrate and document the generalizations. While the text assumes completion of an introductory course in organic chemistry, it reviews the fundamental concepts for each topic that is discussed. The Fourth Edition updates certain topics that have advanced rapidly in the decade since the Third Edition was published, including computational chemistry, structural manifestations of aromaticity, enantioselective reactions and lanthanide catalysis. The two parts stand alone, although there is considerable cross-referencing. Part A emphasizes quantitative and qualitative description of structural effects on reactivity and mechanism. Part B emphasizes the most general and useful synthetic reactions. The focus is on the core of organic chemistry, but the information provided forms the foundation for future study and research in medicinal and pharmaceutical chemistry, biological chemistry and physical properties of organic compounds.
язык: –убрика: ’ими€ /—татус предметного указател€: √отов указатель с номерами страниц ed2k: ed2k stats »здание: 4th edition√од издани€: 1938 оличество страниц: 965ƒобавлена в каталог: 16.04.2006ќперации: ѕоложить на полку |
—копировать ссылку дл€ форума | —копировать ID
ѕредметный указатель
Halides, aryl, copper-catalyzed coupling 495Ч498 Halides, aryl, nickel-catalyzed coupling of 527 Halides, aryl, nickel-catalyzed coupling with Grignard reagents 528 Halides, aryl, palladium-catalyzed alkenylation of 503Ч507 Halides, aryl, palladium-catalyzed coupling with alkenyl stannanes 511Ч514 Halides, aryl, palladium-catalyzed coupling with alkyl boranes 515Ч519 Halides, aryl, palladium-catalyzed coupling with arylboronic acids 515Ч519 Halides, aryl, palladium-catalyzed reactions with organozinc compounds 509 Halides, aryl, preparation from diazonium intermediates 717Ч721 Halides, reductive dehalogenation of 280 283Ч284 288Ч290 296 Halogenation, aromatic 695Ч699 Halogenation, of acid halides 220 Halogenation, of alkenes 202Ч205 Halogenation, of alkynes 225Ч226 Halogenation, of ketones 216Ч220 Halogenation, reagents for 209Ч210 Halogenation, stereoselectivity of 201Ч202 Hard-soft-acid-base theory, application to enolate alkylation 25 Heck reaction 503Ч507 Heck reaction, intramolecular in Taxol synthesis 885 Hexamethylphosphoric triamide (HMPA), solvation by 21Ч25 149 280 389 438 HMPA see УHexamethylphosphoric triamideФ Hofmann Ч Loeffler reaction 655 Hofrnann rearrangement 646Ч648 Homoenolate anion, synthetic equivalents for 841 Horner Ч Wittig reaction 117 Hunsdiecker reaction 793 Hydrazones, chiral, enantioselective alkylation of 38 Hydrazones, diazo compounds from 621 Hydrazones, hydrolysis to ketones 38 Hydrazones, N,N-dimethyl, alkylation of anions of 38 Hydrazones, radical addition to 666 Hydrazones, sulfonyl, diazo compounds from 623 Hydrazones, sulfonyl, Shapiro reaction of 309Ч310 Hydrazones, Wolff Ч Kishner reduction of 307Ч308 Hydroboration 226Ч232 Hydroboration, catalysis of 229Ч230 Hydroboration, enantioselective 230 236Ч238 Hydroboration, of 1,5-dienes 871 Hydroboration, of alkynes 239Ч240 Hydroboration, regioselectivity of 226Ч227 Hydroboration, stereoselectivity of 227Ч228 Hydroboration, thermal reversibility of 230Ч232 Hydroformylation 529Ч530 Hydrogen atom donors 208Ч209 290 314 658 664 716 Hydrogen peroxide, in epoxidation 770 Hydrogenation 249Ч261 Hydrogenation, by dimide 262 Hydrogenation, catalysts for homogeneous 253Ч259 Hydrogenation, enantioselective 253Ч259 Hydrogenation, isomerization during 250 Hydrogenation, mechanism of 250 Hydrogenation, stereoselectivity of 252 Hydrogenolysis 260 Hydrosilation 563 567 Hydroxymethylene derivatives, synthesis of 109 Hypohalites, acyl, as halogenating agents 698Ч699 Hypohalites, ions, in oxidation of methyl ketones 803 Imidazolides see УAcyl imidazolidesФ Imides, reduction of 99 Imines, addition reactions of 96Ч100 Imines, anions, alkylation of 31Ч37 Imines, anions, alkylation of, enantioselective 37Ч38 Imines, anions, alkylation of, regioselectivity of 37Ч38 Imines, formation by rearrangement of alkyl nitrenes 644 Imines, reactions with unsaturated silanes 574Ч575 Imines, reduction by sodium cyanoborohydride 269 Iminium ions, reactions with unsaturated silanes 574 Imino ethers, synthesis of 156 Iodides see УHalidesФ Iodides, alkenyl, from alkynes via hydroboration 239Ч240 Iodides, alkyl, preparation from alcohols 146 Iodides, alkyl, reduction by hydride donors 283Ч284 Iodides, aryl, preparation, by halogenation 688Ч689 Iodides, aryl, preparation, from diazonium ions 719 721 Iodination, aromatic 688Ч689 Iodine azide, as reagent 217 Iodine isocyanate, as reagent 217 Iodine nitrate, as reagent 217 Iodine thiocyanate, as reagent 217 Iodolactonization 205Ч207 Iridium catalysts for homogeneous hydrogenation 253 Isobenzofurans, as Diels Ч Alder dienes 347 Isoxazoles, from alkenes and nitrile oxides by cycloaddition 365 Isoxazolines, from alkenes and nitrones by cycloaddition 364Ч365 Jones reagent 748 Julia Ч Lythgoe alkene synthesis 314 Juvabione, synthesis of 848Ч859 Ketals, alkenyl, as dienophiles 343Ч344 Ketals, as protective groups 822Ч824 829 835Ч836 Ketenes, as intermediates in diazoketone rearrangements 641Ч642 Ketenes, dienophilic synthetic equivalent for 341Ч342 Ketenes, [2 + 2]cycloaddition reactions of 367Ч369 Ketones, 796 Ketones, 779 Ketones, 298 Ketones, 458 Ketones, 276 Ketones, 391 Ketones, 462 Ketones, 216Ч218 Ketones, 219 Ketones, 621Ч622 Ketones, 556 Ketones, 632Ч633 636Ч637 Ketones, 641Ч642 Ketones, 219Ч220 Ketones, 609Ч611 Ketones, 779 Ketones, 555Ч556 Ketones, 462 Ketones, 305Ч306 779 796Ч798 800 Ketones, 276Ч277 Ketones, 487Ч493 Ketones, 521Ч523 Ketones, 39Ч45 89Ч95 Ketones, 5Ч10 Ketones, 9Ч10 26 Ketones, 767 Ketones, 58Ч60 Ketones, 465 Ketones, 372Ч374 Ketones, 580 584 Ketones, 453 Ketones, 122Ч126 Ketones, 11 272Ч273 292Ч293 Ketones, 489Ч490 Ketones, 11 Ketones, 598 Ketones, acylation 108Ч109 Ketones, Baeyer Ч Villiger oxidation of 798Ч800 Ketones, conversion to carboxylic acids by haloform reaction 803 Ketones, enantioselective reduction 278Ч280 Ketones, enantioselective synthesis of 36Ч38 493Ч494 553Ч554 Ketones, enolates, stereoselective formation 5Ч10 Ketones, halogenation of 216Ч219 Ketones, hindered, reaction with organocerium reagents 467 Ketones, hindered, reduction by Grignard reagents 447 Ketones, hindered, Wittig reaction of 112 Ketones, oxidation of 794Ч803 Ketones, oxidation of, 798 Ketones, oxidation of, Cr(VI) reagents 794Ч795 Ketones, oxidation of, lead tetraacetate 796 Ketones, oxidation of, N-sulfonyloxaziridines 797Ч798 Ketones, oxidation of, peroxy acids 798Ч800 Ketones, oxidation of, selenium dioxide 802 Ketones, photocycloaddition reactions of 372 374Ч376 Ketones, preparation from, 794 Ketones, preparation from, acid chlorides and Grignard reagents 451 Ketones, preparation from, acid chlorides and organocadmium reagents 464 Ketones, preparation from, acid chlorides and organocopper reagents 485 Ketones, preparation from, acid chlorides and stannanes 525 Ketones, preparation from, alcohols by oxidation 747Ч757 Ketones, preparation from, alkenes by hydroboration-oxidation 232Ч235 237Ч238 Ketones, preparation from, alkenes by palladium-catalyzed oxidation 501 Ketones, preparation from, alkenyl silanes and acid chlorides 568 Ketones, preparation from, alkenyl silanes by epoxidation 780 Ketones, preparation from, alkyl halides by carbonylation 522 Ketones, preparation from, alkynes by hydration 224Ч225 Ketones, preparation from, aminomethyl carbinols by rearrangement 608 Ketones, preparation from, aromatics by Friedel Ч Crafts acylation 704Ч710 Ketones, preparation from, carboxylic acids and organolithium reagents 453Ч456 Ketones, preparation from, epoxides by Lewis acid-catalyzed rearrangement 778 Ketones, preparation from, nitrites and Grignard reagents 449Ч450 Ketones, preparation from, organoboranes by carbonylation 550Ч555 Ketones, protecting groups for 835Ч837 Ketones, reactions of, with, allylic silanes 568Ч571 574 Ketones, reactions of, with, azides 650 Ketones, reactions of, with, diazoalkanes 608Ч609 Ketones, reactions of, with, hydrazoic acid 649 Ketones, reactions of, with, organolithium compounds 453Ч456 Ketones, reactions of, with, organomagnesium compounds 446Ч450 457Ч458 Ketones, reactions of, with, sulfur ylides 122Ч126 Ketones, reduction by, dissolving metals 292Ч293 299Ч305 Ketones, reduction by, Grignard reagents 447 Ketones, reduction by, hydride exchange 287Ч288 Ketones, reduction by, hydride-donor reagents 262Ч267 273Ч280 Ketones, reduction by, silanes 286Ч287 Ketones, reductive coupling of 299Ч305 Ketones, reductive deoxygenation of 307Ч310 Ketones, ring expansion of cyclic 608Ч609 Ketones, stereoselective reduction of 273Ч280 Knoevenagel condensation 100Ч101 Lactams, by iodocyclization of O,N-trimethylsilyl imidates 207 Lactones, 98 Lactones, formation of 170Ч171 522 636 655 659 801 Lactones, macrocyclic 171Ч172 Lactones, protection as dithioketals 838 Lactones, reduction of 266 Lanthanide salts, as catalysts, 1,3-dipolar cycloaddition 365 Lanthanide salts, as catalysts, addition reactions of allylic silanes 570 Lanthanide salts, as catalysts, alcohol acylation 167 Lanthanide salts, as catalysts, aromatic nitration 697 Lanthanide salts, as catalysts, Baeyer Ч Villager reaction 799 Lanthanide salts, as catalysts, conjugate addition 45 Lanthanide salts, as catalysts, Diels Ч Alder reaction 339 350 Lanthanide salts, as catalysts, ene reactions of aldehydes 401 Lanthanide salts, as catalysts, epoxide ring opening 775 Lanthanide salts, as catalysts, Friedel Ч Crafts reactions 704 Lanthanide salts, as catalysts, Fries rearrangement 710 Lanthanide salts, as catalysts, hydride transfer 287Ч288 Lanthanide salts, as catalysts, Mukaiyama reaction 79 Lanthanides, organo- compounds 467Ч468 Lead tetraacetate, amides, oxidation of 649 Lead tetraacetate, diols, cleavage of 791 Lead tetraacetate, oxidative cyclization of alcohols by 655Ч656 Lead tetraacetate, oxidative decarboxylation of carboxylic acids 792Ч794 Lewis acid catalysis for, addition of diazo compounds to ketones 609 Lewis acid catalysis for, addition of silanes to aldehydes 568Ч573 Lewis acid catalysis for, addition of stannanes to aldehydes 580Ч585 Lewis acid catalysis for, aromatic halogenation 697 Lewis acid catalysis for, Diels Ч Alder reactions 336Ч338 349Ч350 355 Lewis acid catalysis for, dioxolane formation 835 Lewis acid catalysis for, ene reactions 401Ч403 Lewis acid catalysis for, Friedel Ч Crafts reactions 697Ч711 Lewis acid catalysis for, Mukaiyama reaction 79 82 Lindlar's catalyst 260 Lithium tri-t-butoxyaluminum hydride 267 Lithium trialkylborohydrides, as reducing agents 267 276 278 Lithium triethylborohydride 284 776 Lithium, organo- compounds, alkenyl, alkylation of 445 Lithium, organo- compounds, alkenyl, preparation by Shapiro reaction 444 885 Lithium, organo- compounds, alkylation of 445Ч446 Lithium, organo- compounds, alkynyl 438 Lithium, organo- compounds, allylic, alkylation of 445 Lithium, organo- compounds, benzylic, alkylation of 445 Lithium, organo- compounds, configurational stability of 442 Lithium, organo- compounds, cyclization of 452 Lithium, organo- compounds, organoboranes from 548 Lithium, organo- compounds, preparation of 436Ч437 Lithium, organo- compounds, preparation of, by lithiation 438Ч441 Lithium, organo- compounds, preparation of, from halides by halogen-metal exchange 442Ч443 Lithium, organo- compounds, preparation of, from hydrazones by Shapiro reaction 444 885 Lithium, organo- compounds, preparation of, from stannanes by metal-metal exchange 443Ч444 Lithium, organo- compounds, preparation of, from sulfides by reduction 437 Lithium, organo- compounds, reaction with, carbonyl compounds 447Ч449 457Ч458 Lithium, organo- compounds, reaction with, carboxylic acids 453 Lithium, organo- compounds, reaction with, halostannanes 579 Lithium, organo- compounds, reaction with, N-methoxy-N-methylamides 456Ч457 Lithium, organo- compounds, reaction with, trimethylsilyl chloride 563Ч566 Lithium, organo- compounds, structure of 438Ч439 Lombardo's reagent 462 Longifolene, synthesis of 859Ч869 Magnesium, organo- compounds, alkylation of 446 486Ч487 Magnesium, organo- compounds, alkynyl 438 Magnesium, organo- compounds, copper-catalyzed conjugate addition of 494Ч497 Magnesium, organo- compounds, cyclopropylmethyl, ring-opening of 452 Magnesium, organo- compounds, mixed copper reagents 495 Magnesium, organo- compounds, nickel-catalyzed coupling 528 Magnesium, organo- compounds, organoboranes from 548 Magnesium, organo- compounds, preparation of 434Ч435 438 Magnesium, organo- compounds, reactions with, acid chlorides 451 Magnesium, organo- compounds, reactions with, aldehydes 446Ч450 Magnesium, organo- compounds, reactions with, amides 451 Magnesium, organo- compounds, reactions with, carbon dioxide 451 Magnesium, organo- compounds, reactions with, esters 447Ч448 Magnesium, organo- compounds, reactions with, halostannanes 579 Magnesium, organo- compounds, reactions with, ketones 446Ч450 457Ч458 Magnesium, organo- compounds, reactions with, nitrites 450 Magnesium, organo- compounds, reactions with, triethyl orthoformate 451 Magnesium, organo- compounds, reactions with, trimethylsilyl chloride 563 566 Magnesium, organo- compounds, stereochemistry of 435Ч436 Magnesium, organo- compounds, structure of 434 436 452 Magnesium, organo- compounds, unsaturated, isomerization of 451Ч452 Malonate ester anions acylation of 105 108 Malonate ester anions acylation of, alkylation of 11Ч13 Malonate ester anions acylation of, as enolate synthetic equivalents 13 Malonate ester anions acylation of, cyclization of 13 Malonate ester anions acylation of, reaction with 510 Malonic acids, decarboxylation of 13 Malonic acids, in Knoevenagel condensation 101 Mannich reaction 96Ч99 Markownikoff's rule 191Ч192 Meerwein arylation reaction 722 Meerwein Ч Pondorff Ч Verley reduction 287 Mercurinium ion intermediate 196 Mercury compounds, organo- 464Ч465 Mercury compounds, organo-, 659 Mercury compounds, organo-, aromatic 711Ч713 Mercury compounds, organo-, carbenes from 625 633 Mercury compounds, organo-, preparation of 196Ч200 464Ч465 659 Mercury compounds, organo-, reactions of 465 Mercury compounds, organo-, reduction by sodium borohydride 196Ч198 659 Mercury salts in, aromatic halogenation 698 Mercury salts in, aromatic mercuration 711Ч713 Mercury salts in, initiation of polyene cyclization by 600 Mercury salts in, oxymercuration reactions 196Ч200 Mercury salts in, ring-opening of cyclopropanes by 856 Michael reaction see УConjugate additionФ Michaelis Ч Arbuzov reaction 158 Mitsunobu reaction, in nitrogen alkylation 157 Mitsunobu reaction, in preparation of alkyl azides 151 Mitsunobu reaction, in preparation of alkyl iodides 146 Mitsunobu reaction, inversion of alcohol configuration by 153Ч154 Mukaiyama reaction 78Ч82 Mukaiyama reaction, in synthesis of Taxol 887 Mukaiyama reaction, intramolecular in synthesis of longifolene 868 N-bromosuccinimide, aromatic bromination 697 N-bromosuccinimide, bromination of ketones by 216Ч219 N-bromosuccinimide, bromohydrins from alkenes by 203
–еклама
 |
|
ќ проекте
|
|
ќ проекте



 -acetoxy, by oxidation with lead tetraacetate
-acetoxy, by oxidation with lead tetraacetate  -unsaturated, addition of organocopper reagents to
-unsaturated, addition of organocopper reagents to 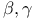 -unsaturated by alkene arylation
-unsaturated by alkene arylation  pyridine HMPA
pyridine HMPA 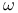 -haloalkyl
-haloalkyl  -allylpalladium compounds
-allylpalladium compounds