|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Pettifor D.G. — Bonding and structure of molecules and solids |
|
|
 |
| Предметный указатель |
Secular equation, NFE 119
Secular equation, OPW 123
Secular equation, TB 86 101 176—177
Selenium 4
Self-consistency 51 64 194
Self-consistent field approximation 45—46
Shape parameter see Moments
Shear constant of semiconductors 206
Shell see also Local coordination polyhedra
Shell closed versus open 131
Shell closing and magic numbers 110
Shell, octet 5
Sigma bonds see Bond integrals
Sigma states of dimer 54 71 72
Sigma versus pi bonds 73
Silicon, band gap 199
Silicon, binding energy curves 47
Silicon, bond order 205
Silicon, dimer 77 73
Silicon, structure 4 5 10 219
Silver atomic energy levels 45
Silver, band parameters 183
Silver, energy bands 82
Silver, equilibrium bulk properties 130
Simple cubic structure see also Structure types
Simple cubic structure, relative structural stability 215—223
Simple hexagonal structure, relative stability of 215—223
Size of atom see Atomic size
Size of core see Core radii
Skewness of eigenspectrum see Moments
Sodium chloride structure see Structure types
Sodium, band width 33
Sodium, binding energy curve 128
Sodium, clusters 111 112
Sodium, density of states 125 126
Sodium, equilibrium bulk properties 130
Sodium, molecules and structure 18
Sodium, pair potential 154 159
Sodium, screening cloud 144
Sodium, structural stability 161—162
Sp atomic energy level splitting 70 74
Sp-valent elements see Structural trends
Sp-valent metals see Structural trends
Space lattice see Bravais lattice
Spherical harmonics 36 40—41
Spin of electron 30
Spin polarized solution 62—64
spin quantum number 36
Stacking sequence 3
Stationary state 30
Stoner band theory of magnetism 227
Stoner criterion for ferromagnetism 228
Stoner exchange integral 63 227
Strontium atomic energy levels 45
Strontium density of states 126
Strontium, equilibrium bulk properties 130
Strontium, structure 3
Structural energy difference theorem and 8-N rule 212
Structural energy difference theorem and s-valent molecules 88
Structural energy difference theorem and sp-valent elements 214—215
Structural energy difference theorem, derivation 83
Structural energy difference theorem, illustrative example 84
Structural energy for pd-bonded AB compounds 238
Structural energy for s-, p-, and sp-valent elements 217 218
Structural trends within 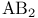 trimers 17—19 100—106 trimers 17—19 100—106
Structural trends within AB compounds 16—17 232—238
Structural trends within lanthanides 12 225—226
Structural trends within s-valent molecules 88—96
Structural trends within sp-valent elements 214—223
Structural trends within sp-valent metals 12 156—166
Structural trends within transition metals 12 223—225 240
Structure factor, definition 146
Structure maps,  163 164 163 164
Structure maps, (\chi_p, \chi_d) 234
Structure maps, Pettifor 12—18 18 end
Structure maps, Phillips — Van Vechten 58
Structure types see also Individual entries
Structure types  -manganese 7 12 -manganese 7 12
Structure types of AB compounds end of book
Structure types,  -tin 47 -tin 47
Structure types, arsenic 4 5 9
Structure types, black phosphorus 9
Structure types, body-centred cubic 3 5 12 17 47
Structure types, caesium chloride 15 16 17 47 235
Structure types, chromium boride 15 16 17 47 235
Structure types, copper-gold (CuAu) 15 47
Structure types, diamond 4 5 17 47
Structure types, face-centred cubic 3 5 7 12 17 47
Structure types, graphite 5 7
Structure types, hexagonal close-packed 3 5 7 12 17 47
Structure types, iron boride 15 16 17 235
| Structure types, iron silicide 15 16 235
Structure types, lanthanum 3 5 12 225
Structure types, manganese phosphide 15 16 235
Structure types, nickel arsenide 15 16 17 235
Structure types, samarium 3 5 12 225
Structure types, simple cubic 5 9
Structure types, sodium chloride 15 16 17 57—59 235
Structure types, sodium thallium (NaTl) 15 17
Structure types, titanium arsenide 12
Structure types, titanium copper  15 17 15 17
Structure types, wurtzite 15 17 57—59
Structure types, zinc blend 15 17 57—59
Sulphur 4
Symmetric versus antisymmetric 55 102
Symmetry see also Bravais lattice
Symmetry even versus odd 41
Symmetry gerade versus ungerade 41 69 71 102—105
Symmetry of Brillouin zone k points 118
Symmetry of NFE states 121
Symmetry translational 5
Symmetry, cylindrical 68
Symmetry, spherical 36
Technetium, band parameters 183
Technetium, bulk properties 189
Technetium, energy bands 182
Tellurium 4
Tetragonal distortion 9 15 150
Tetragonal shear constant 206
Thallium, equilibrium bulk properties 130
Thallium, structural stability 164
Thallium, third moment see Moments
Thomas — Fermi approximation 136—139
Thomas — Fermi screening length, definition 139
Three-centre integrals 86 174
Tight binding approximation and 8-N rule 209
Tight binding approximation for 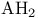 trimers 101 trimers 101
Tight binding approximation for d band 177—178
Tight binding approximation for p band 176—177
Tight binding approximation for s band 173—175
Tight binding approximation for s-valent molecules 85
Tight binding approximation for sp band 214—223
Tin, band gap 199
Tin, bond order 205
Tin, structure 4 5 219
Transition metal aluminides, binding energy curves 48
Transition metals, band structure 180
Transition metals, density of states 181
Transition metals, electronic heat capacity 182
Transition metals, equilibrium bulk properties 189
Transition metals, heats of formation 198
Transition metals, nature of bonding 180—198
Transition metals, structure 12
Triatomic molecules see Molecules
Tungsten, cohesive energy of 10
Uncertainty principle see Heisenberg’s uncertainty principle
Unimodal versus bimodal behaviour see Moments
Unit cell 116
UNITS xi
Unsaturated bond 97 132—133 188 see
Vacancy formation energy 133—134
Valence band width 202
Valence bond theory of 8-N rule 209
Valence bond theory of dimer 60 61
Van der Waals forces 4
Van Hove singularities for fcc, bcc, hep copper 169 170
Van Hove singularities for simple cubic s band 175
Vibrational frequencies of dimers 69
Virtual crystal approximation, definition 195
Walsh diagram for 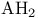 trimer 102 103 trimer 102 103
Wave packet 26 27
Wave-particle duality 20—28
Wigner — Seitz cell, definition 127
Wigner — Seitz radius for 4d metals 183 189
Wigner — Seitz radius for sp-valent metals 130
Wigner — Seitz radius in lerms of core radius 129
Wigner — Seitz sphere, definition 128—129
Wolfsberg — Helmholtz approximation 65
work function 24
Wurtzite structure see Structure types
Ytterbium 3 40
Yttrium, band parameters 183
Yttrium, bulk properties 189
Yttrium, energy bands 182
Zinc selenide, band gap 57
Zinc selenide, bond order 205
Zinc, atomic energy levels 45
Zinc, density of states 126
Zinc, equilibrium bulk properties 130
Zinc, pair potential 165
Zirconium, band parameters 183
Zirconium, bulk properties 189
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



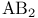 trimers
trimers 
 -manganese
-manganese  -tin
-tin 
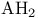 trimers
trimers