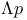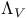|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Truesdell C.A. — The Tragicomical History of Thermodynamics, 1822-1854 |
|
|
 |
| Предметный указатель |
"Maxwell relations 42 114 283 286
"MAYER'S assertion" 157 160
Absolute temperatures see "Temperatures absolute"
Absurdities 100 140 141 297 334
Adiabatic process, definition 33
Adiabatic process, in CARNOT'S theory 123
Adiabatic process, LAPLACE - POISSON law for ideal gases, derivations 38 39 42-46
Adiabatic process, LAPLACE - POISSON law for ideal gases, disregarded by CARNOT 132 133
Adiabatic process, LAPLACE - POISSON law for ideal gases, incorporated by CLAUSIUS 194 198
Adiabats 41 87 264 278 280-282
Air thermometer see "temperature air-thermometer"
Amontons, Guillaume (1663-1705) 10 48 62 183 196 202 342
Ampere, Andr - Marie (1775-1836) 20 108 115 198 206 207 210 299 329 - Marie (1775-1836) 20 108 115 198 206 207 210 299 329
Anomalous behavior of water 13 57 58 267-270
Avogadro, Amedeo (1776-1856) 12 322
B Rard, Jacques Rard, Jacques 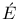 Tienne (1789-1869) 121 126 127 129 133 134 271 272 274 275 276 Tienne (1789-1869) 121 126 127 129 133 134 271 272 274 275 276
Bernoulli, Daniel (1700-1782) 11 47 96 135 152
Bernoulli, Jakob (1654-1705) [?]96
Bernoullis, Family And School 55 63 217
Bharatha, Subramanyam (1945-) Vii 5 13 42 46 58 87 105 108 112 116 122 151 191 196 200 228 232 239 249 270 271 282 292 293 296 297 298 303 327 360
Biot, Jean - Baptiste (1774-1862) 10 29-33 37 44 48-51 60 62 63 87 341-343
Black, Joseph (1728-1799) 16 17 342
Bourbaki, N. [For J. D. (1906-) And A. W. (1906-)]
Bowditch, Nathaniel (1773-1838) 41 342
Boyle, Robert (1627-1691) 11 13 14 177 183 196
Brandes, Heinrich Wilhelm (1777-1834) 11 29 342
Bridgman, Percy Williams (1882-1961) 8 82 100 152 226 338 359
Brown, Sanborn Conner (1913-) 57 103 153 156
BURKHARDT, HEINRICH FrIEDRICH KARL (1861-1914) 77
Callendar, Hugh Longbourne (1863-1930) 129 130
Caloric see "caloric theory of heat" "heat and
Caloric equation of state see "potentials thermodynamic"
Caloric theory of heat (see also "heat functions")
Caloric theory of heat, as adopted by CARNOT 84 85 103
Caloric theory of heat, as adopted by CARNOT, essential to his conclusions regarding finite differences of temperature 107 112
Caloric theory of heat, as adopted by CARNOT, inessential to his conclusions regarding infinitesimal differences of temperature 107
Caloric theory of heat, as adopted by CARNOT, inessential to many of his other conclusions 107 113 130
Caloric theory of heat, as adopted by CARNOT, inessential to the general CARNOT - CLAPEYRON Theorem 111 121 181
Caloric theory of heat, as adopted by FOURIER 59 60 68
Caloric theory of heat, as adopted by LAPLACE 34 35
Caloric theory of heat, as adopted by LAPLACE, inessential to his theory of adiabatic change 46
Caloric theory of heat, as adopted by LAPLACE, inessential to his theory of sound 44
Caloric theory of heat, broad sense 34 153
Caloric theory of heat, forbids ideal gas to have constant specific heats 41 132
Caloric theory of heat, inessential to CARNOT'S general axiom 185
Caloric theory of heat, inessential to some of HOLTZMANN'S conclusions 181-184
Caloric theory of heat, inessential to the HELMHOLTZ- JOULE determination 181
Caloric theory of heat, required by CARNOT'S special axiom 104 105
Calorimetric 19
Calorimetry, theory of 19
Caratheodory, Constantin (1873-1950) Vii
Carlson, Donald Earle (1938-) 146
CARNOT - CLAPEYRON theorem, general 111
CARNOT - CLAPEYRON theorem, special 111 112
CARNOT'S, approach and assumptions 78-85
CARNOT'S, cycle 86-88
CARNOT'S, function 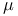 106 108 170 249 106 108 170 249
CARNOT'S, function F 102 103 112
CARNOT'S, general axiom 101
CARNOT'S, heat function 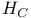 85 85
CARNOT'S, main theorem 110
CARNOT'S, special axiom 102 103
Carnot, Lazare - Hippolyte (1801-1888) 80
Carnot, Lazare - Nicolas- Margu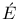 Ute (1753-1823) 135 Ute (1753-1823) 135
Carnot, Nicholas - Leonard - Sadi (1796-1832) Vii 4 5 8 10 11 15 16 18-22 25 42 60 79-143 147 149-151 153-155 158-160 162 164 168- 191 194-206 210 213 215 218- 220 224-229 231-239 241 242 245 248 249 253 269 270 272- 287 288 291-293 297 298 300 303 306 308-312 318 321 322 324-331 333-335 339 344
Cauchy, Augustin - Louis (1789-1857) 15 20 67 69 77 109 134 13 5 217 238
Chladni, Ernst Florens Friedrich (1756-1827) 342
Cl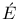 Ment, Nicholas (1778/9-1841) 36 Ment, Nicholas (1778/9-1841) 36
Clairaut, Alexis Claude (1713-1765) 200
Clapeyron, Benoit - Pierre-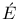 Mile (1799-1864) 20 26 60 80 107 110-116 120 121 124 127 129 134 136 139-142 151 152 155 158 160 162 168 169 171 173 175 177 181 182 184 187-189 195 197- 201 203 206 213 215 227- 229 234-238 242 245 269 270 277-279 292 308 311 318 321 326-331 333 346 Mile (1799-1864) 20 26 60 80 107 110-116 120 121 124 127 129 134 136 139-142 151 152 155 158 160 162 168 169 171 173 175 177 181 182 184 187-189 195 197- 201 203 206 213 215 227- 229 234-238 242 245 269 270 277-279 292 308 311 318 321 326-331 333 346
Clausius, Rudolf Julius Emmanuel (1822-1888) Vii 5 10 11 16 17 20 34 81 104 105 107 112 121 122 127 143 151 156 158-162 171 182 185 187-207 210 213- 219 223-228 230 233-237 241 242 244 245 248 249 258 260 264-266 273-275 277-280 282 284 285 287 292 293 295-300 303 310-320 324 329-335 337 338 349 352 354-357
Compression, sudden see "adiabatic process "
Conduction of heat (see also "conductivity")
Conduction of heat, BIOT'S theory 48-51
Conduction of heat, DUHAMEL'S theory 143-147
Conduction of heat, excluded by CARNOT 82 87 91
Conduction of heat, excluded by classical thermodynamics 338
Conduction of heat, excluded by CLAUSIUS 319
Conduction of heat, FOURIER'S theory 51-53 60-75
Conduction of heat, LAMBERT'S theory 47-49
Conduction of heat, mentioned by KELVIN 305
Conduction of heat, refers essentially to time 22
Conductivity, thermal (see also "NEWTON'S law of cooling")
Conductivity, thermal, specific 60 67
Conductivity, thermal, superficial 60
Conservation of energy see " Energy conservation
Constitutive, constant of an ideal gas 12
Constitutive, functions 19
Constitutive, inequalities 12 16 18 19 23 24 173 267-270
Constitutive, relations 19 67 71 76-78 82 338 339
Constitutive, restrictions imposed by thermodynamics, CARNOT - CLAPEYRON theorems 111-113
Constitutive, restrictions imposed by thermodynamics, CARNOT'S great discovery 134 135
Constitutive, restrictions imposed by thermodynamics, CLAUSIUS 192 199 203 317 318 329
Constitutive, restrictions imposed by thermodynamics, of caloric theory 42 114
Constitutive, restrictions imposed by thermodynamics, RANKINE'S 213 215 216
Constitutive, restrictions imposed by thermodynamics, REECH'S 282 284-286
Continuum mechanics 67 69 70 73 77
Convection 72
Crawford, Adair (1749-1795) 22
CYCLE 86(see also "CARNOT'S cycle")
d'Alembert, Jean le Rond (1717-1783) 77
Dalton, John (1766-1844) 9-11 96 177 342
Dante Alighieri (1265-1321) 1 6 9 29 47 79 139 149 187 219 277 305 337 339
Darboux, Jean - Gaston (1842-1917) 52 58 60 64 66 67 69
Daub, Edward Vii
Davini, Cesare (1943-) Vii
Davy, Humphry (1778-1829) 34
Delaroche, Fran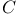 Ois (1775-1813) 121 126 127 129 133 134 271 272 274-276 Ois (1775-1813) 121 126 127 129 133 134 271 272 274-276
Deltas of thermodynamics 3 4 22 199 206
Desormes, Charles - Bernard (1777-1862) 36
Despretz, C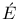 Sar Mansuete (1792-1863) 52 Sar Mansuete (1792-1863) 52
Devil, the 68
Diagrams 20 86 140 264
Differentials 3 7 8 21 22 26 27 140 206 207 210
Dimensions, applied to CARNOT'S theory 118 119
Dimensions, FOURIER'S theory of 65 66
Dissipation see "irreversible processes"
Doctrine of latent and specific heats 16 19 theory
Duhamel, Jean - Marie - Constant (1797-1872) 25 67 68 143-147 168 346
Duhem, Pierre - Maurice - Marie (1861-1916) 338
Dulong, Pierre Louis (1785-1838) 142 175 193 194
Dupre, Athanase (1808-1869) 298
Dynamical theory of heat see "interconvertibility of heat and work uniform
Efficiency (see also " CARNOT'S general axiom")
Efficiency, formal definition by RANKINE 264
Efficiency, KELVIN'S evaluation for a CARNOT cycle 230-232
Efficiency, maximum achieved by CARNOT cycles 90-95 116 117 171 172 302
Efficiency, preliminary definition 103
Efficiency, RANKINE'S evaluation for a CARNOT cycle 221-224
Efficiency, universal 95-99
Empirical temperatures see "temperatures empirical"
Energy (see also "pro-energy" "first "interconvertibility
Energy, balance of 71 246
Energy, conservation of 143 163 259
Energy, in CLAUSIUS' theory 333 334
Energy, in RANKINE'S theory 210 211 214 220-223 264 265
Energy, in REECH'S theory 334
Energy, internal, in CLAUSIUS'S theory 153 189 192 199 200
Energy, internal, in KELVIN'S theory 227 233
Energy, internal, in RANKINE'S theory 213
Energy, internal, in thermomechanics 71
Energy, internal, possibly total heat 15
Energy, kinetic 71 109 a
Energy, potential 259
Energy, science of 164
Energy, total 15 152 161
Engine 79 150 151 164 168 310
Enthalpy 246 247 266 287
entropy (see also "heat function" and "pro-entropy")
EQUIVALENCE see "interconvertibility of heat and work "
| Equivalent see "mechanical equivalent of a unit of heat"
Euler, Leonhard (1707-1783) 11 12 14 15 19 29 47 50-52 55 63 69 70 72 73 96 114 135 135 152 205 208 217 281 342
Expansion, latent heat of see "latent heat with respect to volume"
Experiment see in the index of persons "BOYLE" "AMONTONS" "LAVOISIER" "RUMFORD" "DALTON" "GAY-LUSSAC" "DELAROCHE" "DULONG" "WELTER" "JOULE" "REGNAULT" "MAGNUS"
First law of thermodynamics (see also "interconvertibility of heat and work" "energy" "mechanical and
First law of thermodynamics, CLAUSIUS 105 192 193 199 200 249 311 312 332
First law of thermodynamics, KELVIN'S 227 245
First law of thermodynamics, not known to CLAPEYRON 139
First law of thermodynamics, not known to MAYER, HELMHOLTZ, JOULE or anyone else before 1850 184 185
First law of thermodynamics, not known to RUMFORD 156
First law of thermodynamics, RANKINE'S 213 257 259
First law of thermodynamics, REECH'S generalization 287
Fluid 12
force see "heat a
Fourier, Jean Baptiste Joseph (1768-1830) Vii 9 16 25 27 32 48 49 51-78 79 82 87 119 135-137 143-147 153 174 338 343 346
Fox, Robert 16 17 22 26 31 33 34 40 41 198 271-273
Free energy 287
Free enthalpy 287
Freeman, Alexander (1838-1897) 52 60 343
Freudenthal, Hans (1905-) 20
Furnace 87
Gas 11 14 278 "vapors")
Gauss, Carl Friedrich (1777-1855) 20
Gay - Lussac, Joseph Louis (1778-) 1850 9-11 32 35-37 45 96 126 132 177 190 193 341
Generosity, retrospective 68 267
Gibbs relation 282 299
Gibbs, Josiah Willard (1839-1903) Vii 90 161 179 185 264 265 282 287 299 300 358
Grattan-Guinness, Ivor 52
Green, George (1793-1841) 147
Hadamard, Jacques - Salomon (1865-1963) 338
Heat (see also "heating" "latent "specific "caloric "heat "conduction "interconvertibility "energy" "pro-energy" "first
Heat functions (see also "caloric theory of heat")
Heat functions, CARNOT'S 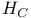 85 123 159 85 123 159
Heat functions, CLAPEYRON'S 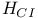 141 141
Heat functions, LAPLACE'S  35 159 35 159
Heat, a kind of force 153-155 162 178
Heat, a manifestation of intestine motion 152 153 188 207 208 210
Heat, absorbed by a body 25 83
Heat, added to a body 15 20
Heat, emitted by a body 25 83
Heat, flux of 61 62 66 67 69 76
Heat, total 15 152 153 178 192
Heating 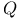 15 246 15 246
HELMHOLTZ - JOULE determination of 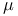 , accepted by Clausius 195-198 201 , accepted by Clausius 195-198 201
HELMHOLTZ - JOULE determination of 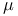 , considered by CARNOT 128 , considered by CARNOT 128
HELMHOLTZ - JOULE determination of 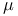 , derived by RANKINE 213 , derived by RANKINE 213
HELMHOLTZ - JOULE determination of 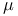 , equivalent to Holtzmann's assertion 181 297 , equivalent to Holtzmann's assertion 181 297
HELMHOLTZ - JOULE determination of 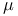 , from dimensional invariance of CARNOT'S Theory 119 , from dimensional invariance of CARNOT'S Theory 119
HELMHOLTZ - JOULE determination of 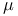 , inferred from HOLTZMANN'S theory by HELMHOLTZ 162 , inferred from HOLTZMANN'S theory by HELMHOLTZ 162
HELMHOLTZ - JOULE determination of 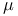 , priority of JOULE contested by CLAUSIUS 231 232 , priority of JOULE contested by CLAUSIUS 231 232
HELMHOLTZ - JOULE determination of 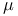 , rejected by Kelvin 174 176-178 231-233 , rejected by Kelvin 174 176-178 231-233
HELMHOLTZ-JOULE determination of 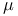 , inferred from HOLTZMANN'S assertion by JOULE 176 180 181 , inferred from HOLTZMANN'S assertion by JOULE 176 180 181
Herakleitos Of Ephesos (Fl. C 500 A.C.) 80
Herapath, John (1790-1868) 45 344
History of science 4 5 32 46 117 295
HOLTZMANN'S assertion (see also "MAYER'S assertion")
HOLTZMANN'S assertion, adopted by JOULE 167
HOLTZMANN'S assertion, called "MAYER'S hypothesis" by KELVIN 161
HOLTZMANN'S assertion, derived by RANKINE 212 215
HOLTZMANN'S assertion, equivalent to MAYER'S 159-161
HOLTZMANN'S assertion, logical status and generalization 181-183
HOLTZMANN'S assertion, statement 158-160
HOLTZMANN'S assertion, support for it implied by HELMHOLTZ'S table 162
Holtzmann, Carl Heinrich Alexander (1811-1865) 158-162 167 177 181-185 188 190 193-198 200 201 202- 212 213 215 232 234 241 242 244 248 249 268 269 273 297 320 326 332 347
Hope, Thomas Charles (1766-1844) 57
HOPPE'S theorem on ideal gases 202 275
Hoppe, Edmund (1854-1928) 52
Hoppe, Ernst Reinhold Eduard (1816-1900) 202 248 249 275 355
Hoyer, Ulrich 156
Hugoniot, Pierre Henri (1851-1887) 14
Hutchison, Keith 108
Huygens, Christiaan (1629-1695) 55 69 70 217
Ideal gas 9-12 96 ideal-gas")
Imhotep (Fl. A.C. 2980-2950) 68
Inequalities see "constitutive inequalities"
Ingen - Housz, Jan (1730-1799) 52
Interconvertibility of heat and work (see also "energy" "proenergy" and
Interconvertibility of heat and work, in cyclic processes, conceptual analysis of 149-151
Interconvertibility of heat and work, in cyclic processes, universal but not uniform 115 116 150
Interconvertibility of heat and work, universal and uniform, according to JOULE 163-167 185
Interconvertibility of heat and work, universal and uniform, according to MAYER 154-157 184 185 267
Interconvertibility of heat and work, universal and uniform, in all circumstances 153 178 184
Interconvertibility of heat and work, universal and uniform, in cyclic processes 189 190 209
Interconvertibility of heat and work, universal and uniform, in general processes see " energy internal"
Interconvertibility of heat and work, universal and uniform, in isothermal processes 154 158-160 176 178
Interconvertibility of heat and work, unknown to RUMFORD 156
Intestine motion see "heat"
Invertibility, thoughtless, principle of 283
Irreversible processes 47-78 91 94 164 167 244 246 305 306 318 319 334 335 338
Isothermal 15
Ivory, James (1765-1842) 38 39 345
James, Richard (1952-) Vii 92
Jochmann, Emil Carl Gustav Georg (1833-1871) 356
John The Grammarian (Labor- Loving) (Fl.  C.) 68 C.) 68
Jones, Tom (Fl. C 1749) 26
JOULE - THOMSON effect 242-244 249-256
Joule's equivalent see "mechanical equivalent of a unit of heat"
Joule, James Prescott (1818-1889) 34 156 162 164-167 174-181 184 185 188 189 195 196 201 203 208 209 213 222 224-227 231-233 235 242-245 249-256 267 272-277 297 309 327 348 350 351 353 354
Kelland, Philip (1808-1879) 26 27 346
Kelvin, Lord (William Thomson) (1824-1907) Vii 8 11 16-21 23 34 58 86 89 107 108 111 112 114 122 124 125 136 147 155 156 159-161 168-178 180 181 184 185 187 188 192 196 199 200 202-205 207-209 219 220 222 224-236 241-256 257 258 263 266 267- 272-274 276-278 285 288 292 295 296 305-310 311 312 320-324 326-335 349-354 356 358
Kirchhoff, Gustav Robert (1824-1887) 217 338
Klein, Christian Felix (1849-1925) 161
Klein, Martin Jesse (1924-) 264 265
Krafft, Jens (1720-1765) 48
Lagrange, Joseph Louis (1736-1813) 12 14 30 55 72 77 140 342
Lamb, Horace (1849-1934) 14
Lambert, Johann Heinrich (1728-1777) 47-49 51 52 62 87 341 342
Lame, Gabriel (1795-1870) 143
LAPLACE - POISSON law see " adiabatic process LAPLACE
Laplace, Pierre Simon de (1749-1827) 11 12 14 16-18 21 26 29-46 50-52 55 58 63 68 75 77 81-85 87 109 114 122 125 126 131-133 135 136 140-142 145 159 196- 198 201 202 204 212 235 247 248 272 273 275 341-344
Latent heat (see also "calorimetry theory
Latent heat, of fusion or vaporisation 17
Latent heat, with respect to pressure 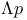 22-24 22-24
Latent heat, with respect to volume 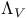 16-19 16-19
Lavoisier, Antoine - Laurent (1743-1794) 18 21 58 82 341
Laws of thermodynamics see "first law" and "second laws"
Legendre, Adrien - Marie (1752-1833) 55
Leibniz, Gottfried Wilhelm (1646-1716) 55
Leslie, John (1766-1832) 52
Line integrals 20 24
Linearity, confusion it leads to 77 78 143-147
Lippmann, Gabriel Jonas (1845-1921) 129 135 136
Lipschitz, Rudolf Otto Sigismund (1832-1903) 161
Lucretius Caro, Titus (A.C., 96-55) 152
Mach, Ernst (1838-1916) 47 156 322 324 359
Magie, William Francis (1858-1943) 187 349
Magnus, Heinrich Gustav (1802-1870) 11
Man, Chi - Sing (1947-) Vii 10 11 41 167 177 179-181 249-256
Mariotte, Edm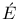 (1620-1684) 36 96 177 190 (1620-1684) 36 96 177 190
Massieu, Fran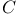 Ois Jacques Dominique (1832-1896) 287 299 300 358 Ois Jacques Dominique (1832-1896) 287 299 300 358
material 20
Materiality of heat see "caloric theory of heat"
Mathematics (see also "differentials")
Mathematics, applied 339
Mathematics, CARNOT'S 80 101 117 124 134 136 168
Mathematics, CLAPEYRON'S 140
Mathematics, CLAUSIUS 205-207 218 311
Mathematics, FOURIER'S 55 66 77
Mathematics, KELVIN'S 168 227 231
Mathematics, LAPLACE'S 46
Mathematics, RANKINE'S 210 211 216 217 265
Mathematics, REECH'S 297 299
Mathematics, special, for thermodynamics 8 26 27 206 217
Maxwell, James Clerk (1831-1879) 9 18 67 70 90 114 146 147 163 185 216 259 264-266 274 283 338 350 358 359
MAYER'S hypothesis" see "HOLTZMANN'S assertion"
Mayer, Julius Robert (1814-1878) 34 122 154-161 163 164 174 180-185 188 189 193 196 198 201 202 204 209 212 224 234 236 242-244 246 248 249 267 269 273 274 277 346 348
Mechanical equivalent of a unit of heat, CARNOT'S calculation 80 81 119-122
Mechanical equivalent of a unit of heat, CLAUSIUS' calculation 204
Mechanical equivalent of a unit of heat, erroneous attribution to RUMFORD 156
Mechanical equivalent of a unit of heat, HOLTZMANN'S calculation 159 182
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



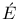 - Marie (1775-1836)
- Marie (1775-1836) 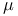
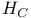
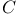 Ois (1775-1813)
Ois (1775-1813) 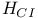

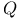
 C.)
C.)