|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Truesdell C.A. — The Tragicomical History of Thermodynamics, 1822-1854 |
|
|
 |
| Предметный указатель |
Mechanical equivalent of a unit of heat, in cyclic processes 150 151 189
Mechanical equivalent of a unit of heat, in isothermal processes 154 158 160 167
Mechanical equivalent of a unit of heat, JOULE'S determination 167
Mechanical equivalent of a unit of heat, MAYER'S calculation 157 182
Mechanical equivalent of a unit of heat, WATERSTON'S calculation 156
Mechanical theory of heat see "interconvertibility of heat and work uniform
Meikle, Henry 38 39 41 42 45 345
Mendoza, Eric 84 139 153
Meyerson,  Mile (1859-1933) 180 Mile (1859-1933) 180
Middleton, William Edgar Knowles (1902-) 10
Moigno, Fran Ois - Napol Ois - Napol On- Marie (1804-1884) 299 On- Marie (1804-1884) 299
Molecular models, CLAUSIUS 205 217 274 275 321
Molecular models, DUHAMEL'S 67 68
Molecular models, in general 10 34
Molecular models, LAPLACE'S 32 33
Molecular models, RANKINE'S 207-209 216 217 274 275
Monge, Gaspard (1746-1818) 77
Motive power see "work" and "efficiency"
Navier, Claude - Louis - Marie- Henri (1785-1836) 77 144
Neumann, Franz Ernst (1798-1895) 144 147 280 347 359
Newton's law of cooling 47 48 50 62
Newton, Isaac (1642-1727) 13 14 46 47 48 50 55 62 63 217
notations 7 8
Oepidus (Fl. [?] Before A.C.700) 26
Oersted, Hans Christian (1777-1851) 96
Operating temperatures of a CARNOT cycle 88
Palmieri, Marco & Giuseppe (Fl 1750) 26
Partington, James Riddick (1886-1965) 23 44 57 196
Path 20 21
Pedersen, Olaf 5
Perpetual motion 95 100 191
Petit, Alexis Th R R Se (1791-1820) 109 135 167 175 247 343 Se (1791-1820) 109 135 167 175 247 343
Philoponos, John (Fl.  C.) 68 C.) 68
Physicists, psychology of 152
Physics and chemistry 59 76 81 135 136 153 295
Pitteri, Mario (1948-) Vii
Poincar , Jules Henri (1854-1912) 179 , Jules Henri (1854-1912) 179
Poisson, Sim On - Denis (1781-1840) 9 11 12 23 26 30 31 33 36-46 53 60 62 63 69 77 109 122 132 133 141-143 145 158 193 194 196 198 201 202 204 235 248 342 344-346 On - Denis (1781-1840) 9 11 12 23 26 30 31 33 36-46 53 60 62 63 69 77 109 122 132 133 141-143 145 158 193 194 196 198 201 202 204 235 248 342 344-346
potential energy 259
Potentials, thermodynamic 285-287 299 300
Power see "working" "work" and
Power, Henry (1623-1668) 13 14 183 196
Pro-energy, in CARNOT'S special theory 116
Pro-energy, REECH'S 282 287 296
Pro-entropy, implied by CARNOT'S general theory 293
Pro-entropy, REECH'S 278-280 333
Process 15 21 83 "line "adiabatic "isothermal" "reversibility "irreversible
Ptolemy (Fl.  C.) 44 C.) 44
Quasistatic 153 279 311 337
Rankine, William John Macquorn (1820-1872) Vii 10 11 17 22 33 34 44 129 180 205 207-224 227 232 233 235 236 248 251 253 256 257-267 274-277 280 298 311 334 350-356
Rate-independence of calorimetry and classical thermodynamics 21 22 24
Rational mechanics 53 77 80 134
Rational thermomechanics 3 4 9 22 26 52 53 67
Ravetz, Jerome R. 51
Rayleigh, John William Strutt, Baron (1842-1919) 14
REECH'S theorem" on the ratio of specific heats 43 289
REECH'S, first theorem 106 239
REECH'S, fourth theorem 285 286
REECH'S, second theorem 241
REECH'S, third theorem 280
Reech, Ferdinand (1805-1884) 1 21 27 43 44 46 81 104 106 112 115 128 129 236-241 247 266 277-301 333 334 337 350 353 355-358
Refrigerator 87
Regnault, Henri Victor (1810-1878) 11 169 173 175 177 179 196 197 203 208 224 237 242 248- 250 252-255 264 266 273 274 276 277 279 297 298 307 308 320 347 349 353 357
Reversibility (see also " irreversible processes")
Reversibility, for CLAUSIUS 313 316
Reversibility, for KELVIN 305
Reversibility, in CARNOT'S theory 94 168
Reversibility, in classical thermodynamics 25 164 337
Reversibility, of heat 21 25
Reversibility, of perfect engines 168
Reversibility, of work 24 25
Richmann, Georg Wilhelm (1711-1753) 48
Riemann, Georg Friedrich Bernhard (1826-1866) 322
Rowland, Henry Augustus (1848-1901) 180 359
Ruffner, James Alan (1930-) 48
Rumford, Benjamin Thompson, Count (1753-1814) 34 48 52 57 62 103 156
| Second laws of thermodynamics, CARNOT'S, alleged 90 100 105
Second laws of thermodynamics, CLAUSIUS 105 190 191 226 312 316 319 333
Second laws of thermodynamics, KELVIN'S 226
Second laws of thermodynamics, RANKINE'S 213 214 262 264-266
Second laws of thermodynamics, various 8 101 134 249
Secondary literature 5 341
Serrin, James Burton, Jr. (1926-) Vii 322 328
Socrates (A.C. 470?-399) 155
Sound, speed of, independent of caloric theory 42-44
Sound, speed of, LAPLACE - POISSON theory disregarded by CARNOT 131-133
Sound, speed of, results established in  century 13 14 century 13 14
Sound, speed of, results of BIOT, POISSON, LAPLACE 29-37
Specific heat, at constant pressure 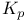 22-24 22-24
Specific heat, at constant volume  16-19 16-19
Specific heats, CARNOT'S theory 122-125
Specific heats, constant difference assumed by MAYER 157
Specific heats, constant ratio rejected by CARNOT 131
Specific heats, constant, excluded by caloric theory 42 125 132
Specific heats, constant, required by RANKINE'S theory 216
Specific heats, constant, suggested by CLAUSIUS 196
Specific heats, FOURIER'S ideas 58 59
Specific heats, LAPLACE'S theory 36 40-42
Specific heats, MEIKLE'S theory 38 39
Specific heats, REECH'S general theory 288-291
State, thermodynamic 81 82 142 337
Steam 79 81 90 121 136 150 168 169 173-175 177 178 185 203-205 231 234 247 272 273 308
Stefan, Josef (1835-1893) 72 357
Stokes, George Gabriel (1819-1903) 68 207 350
Strepsiades (Fl. A.C.423) 149 155
Surroundings 83 98 99
Taylor, Richard 158
Temperatures, absolute 108 307-310 320-322 328-332
Temperatures, air-thermometer 11 249 307
Temperatures, difference, finite 85 105 109 173
Temperatures, difference, infinitesimal 105-107 1109-111 141
Temperatures, difference, makes work possible, CARNOT'S statements 84 89 90 95 178
Temperatures, difference, makes work possible, GIBBS' statement 90
Temperatures, empirical 307 321-328
Temperatures, field 60
Temperatures, FOURIER'S explanation 56 57
Temperatures, ideal-gas 9 323
Thermal equation of state 12 19 theory
Thermo-elasticity 143-147
Thermodynamic potentials see "potentials thermodynamic" "energy internal" "enthalpy" "free "pro-energy" "pro-entropy" "free
Thermomechanics see " rational thermomechanics "
Thermometric axiom 327 328
Thermostatics 299
Thompson, Benjamin see Rumford
Thomson, James (1822-1892) 86
Thomson, William see Kelvin
Time, essential to thermodynamics 15 22
Time, obscured by CARNOT 136 137
Time, rarely mentioned 22
Toupin, Richard A. (1926-) 14 68 277 289 359
Towneley, Richard (1629-1707) 13 14 183 196
Traditional presentations of thermodynamics 3 6
Truesdell, Clifford Ambrose, Iii (1919-) Vii 4 5 10 13 14 41 42 46 58 81 87 105 108 112 116 122 152 156 171 172 191 196 214 228 232 239 249 270 277 280 282 289 292 293 296-298 303 322 327-329 359 360
Universe 338
Unpublished sources 5
V. Helmholtz, Hermann Ludwig Ferdinand (1821-1894) 107 155 160 161-163 177 182 185 189 195 198 201 204 207 209 213 222 232 233 235 242 244 245 266 272- 295-297 327 348 354-356
van der Waals fluid 211
Vapors 11 192 195 278
Variables, unfortunate choice of 140
Vis viva see "heat a
Water see "anomalous behavior of water"
Waterston, John James (1811-1883) 34 44 153 156 300
Watt, James (1736-1819) 264
Weber, Wilhelm Eduard (1804-1891) 23 345
Welter, Jean Joseph (1763-1852) 32 36 37 45 126 132 193
Whewell, William (1794-1866) 198
Winters, Stephen (1946-) Vii
Work (see also "reversibility of work" and "energy internal")
Work, CARNOT'S great contribution 135
Work, definition 24
Working 246 (see also "work")
Y, Charles (1814-1889) 8
Zeuner, Gustav Anton (1828-1907) 298 356
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



 Mile (1859-1933)
Mile (1859-1933)  Ois - Napol
Ois - Napol Se (1791-1820)
Se (1791-1820)  C.)
C.)  C.)
C.)  century
century