|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Kubo R. — Thermodynamics |
|
|
 |
| Предметный указатель |
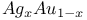 242 282 242 282
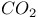 , Joule — Thomson coefficient 109 , Joule — Thomson coefficient 109
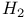 , enthalpy 72 , enthalpy 72
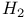 , entropy 72 , entropy 72
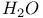 37 90 114 228 238 271 37 90 114 228 238 271
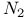 33 33
 -phase 216 -phase 216
 -phase 216 -phase 216
Absolute temperature 10 11 65 88 93 121
Absolute zero 140 159 182
Activity 202
Activity coefficient 202 226
Additivity of entropy 74
Adiabatic bulk modulus 31
Adiabatic compressibility 23 88 103 161
Adiabatic demagnetization 91 113 164
Adiabatic expansion 100 161 249
Adiabatic expansion, temperature decrease 109
Adiabatic irreversible process 5
Adiabatic Joule — Thomson effect 161
Adiabatic magnetic susceptibility 35 89 104 164 182
Adiabatic process 4 249
Adiabatic temperature coefficient 160
Adiabatics 85 97
Air 20 33 47
Air, enthalpy 149
Air, Joule — Thomson coefficient 94
Air, specific heat 152 153
Amphoteric electrolyte 280
Argon 16
Arrhenius' theory 205
Atmosphere of some stars 226
Atmospheric pressure 41
Average diameter of the ions 204
Azeotropy 239
Barometer equation 42
Boiling point 233 256
Boiling point elevation 218 219
Boltzmann's constant 13 264
Boyle's curve 25 26 27 28
Calorie 8 see
Caratheodory's principle 62 92 115
Carnot's cycle 44 62 65 113
Carnot's principle 64
Centigrade temperature scale 11
Chemical constant 173 197 210 263
Chemical equilibrium 205 283
chemical potential 9 138
Chemical potential, gas 197 198
Chemical reaction 205 283
Chemical reaction, advancement 260
Chemical reaction, degree of advancement 283
Chemical reaction, enthalpy 205 235
Chemical reaction, free energy 205 209 235
Chemical reaction, standard enthalpy 206 235 261
Chemical reaction, standard free energy 206 209
Chemical reaction, standard internal energy 261
Clausius — Clapeyron relation 191 245 246 256 268 271
Clausius' equality 63
Clausius' equation 32 54
Clausius' inequality 69
Clausius' principle 63 78 85 115
Closed system 1
Coefficient of thermal expansion 269
Coexistence of the two phase 190 212
Coil 12
Colloidal solution 280
Concentration 202
Condensation of water vapor and an electric charge 290
Condensed phase 214
Contact potential 227
Conventional chemical constant 263
Corresponding state 25 26 33
Critical field, magnetic 238
Critical radius of liquid drop 271 290
Critical state 25 45
Critical temperature 26 210 236
Cu-Ni alloy 233
Curie temperature 243 289
Curie — Weiss law 93 177 182 243
Curie's constant 16
Curie's law 16 84 91 164 182
CYCLE 4
Daniel cell 207 208 226
Debye — Hueckel's limitting law 204 241
Degree of dissociation 226 234
Degree of freedom 195
Demagnetizing field 18 59
Dependence on the process 7
Dielectrics 165 184 185
Diesel's cycle 87
Dietrici's equation of state 33 45 94 124 125
Diffusion 92 240
Dilute solution 200 257
Direction of actual change 75
Dissociation 203 235 262
Dissociation pressure 235
Distillation 239
Donnan's membrane equilibrium 240 280
Duhem — Margules relation 253
Efficiency 64 85
Electric displacement 185
Electric polarization 187
Electrical neutrality, condition of 203
Electrochemical cell 207 235
Electrochemical potential 209
Electrode reaction 207
Electromotive force 208 226 242
Empirical temperature scale 11
Endothermic reaction 284
Energy 3 5 147
Enthalpy 8 28 56 147 160
entropy 70 71 74 136 147 160
Entropy of melting 258
Entropy of mixing 82 195 198
Entropy-temperature diagram 111
Environment 1
Equation of continuity 56
Equation of state 12 57 88
Equation of state, ideal gas 13
Equilibrium constant 206 210 234 261
Equilibrium pressure curve 246
Eutectic point 216 233 258
Existence theorem of temperature 37 57
Exothermic reaction 284
Expansion coefficient 93
Extensive property of Helmholtz free energy 175
Extensive quantity 3
Extensive Variable 230
External variable 3
Faraday's constant 208
Ferromagnetic substance 288
Ferromagnetic substance, specific heat 243 289
First law of thermodynamics 5 56
Fixed point of temperature 68
Free energy 147
Free energy, a sping and a weight 183
Free energy, dielectrics 185 187
Free energy, paramagnetic substance 164
Free enthalpy 147
Free expansion 32 43 87
Freezing point 257
Freezing point depression 218 219 257
Freezing point elevation 257
Fugacity 198 202 206 231
Gas constant 13
Gas mixture 231
Gas phase 209
Gas thermometer 11 65 126
Gas thermometer, constant pressure 94
| Gaseous line 239 274
Gay-Lussac's experiment 91
Gebundene Energie 147
Gibbs — Duhem relation 137 162 180 200 212 224 231 240 252
Gibbs — Helmholtz equation 94
Gibbs — Poynting relation 192
Gibbs' free energy 84 136 137 160 173 174
Gibbs' phase rule 195
Gibbs' relation 71
Gold 242
Grand potential 136
Heat 3 5
heat capacity 11 19 137 271
Heat capacity at constant chemical potential 163
Heat capacity at constant electric displacement 185
Heat capacity at constant electric field 185
Heat capacity at constant magnetization 164
Heat capacity at constant pressure 11 35 65 106 150 151 154
Heat capacity at constant volume 35 106 137 150 154
Heat capacity for constant magnetic field 35 164
Heat capacity, negative 269
Heat capacity, non-negativity 156
heat engine 62
Heat function 147 see
Heat of fusion 209 257
Heat of transition 191
Heat of vaporization 209 214 215
Heat radiation 52 53 80
Heat reservoir (heat bath) 3 62 69 76
Helmholtz free energy 76 136 147 159 160 164 167 177
Helmholtz free energy of the surface 214
Henry's law 200 231 255
High-polymer solution 240 243 277 278 292
Hydrogen chloride 181 241
Ice point 66 233
Ideal dilute solution 201 203 233
Ideal gas mixture 14 45 162 173 198
Ideal gas, chemical potential 157 165 198
Ideal gas, enthalpy 34
Ideal gas, entropy 87 101 156 160
Ideal gas, equation of state 13
Ideal gas, Gibbs' free energy 106 156 160
Ideal gas, Helmholtz free energy 106 157
Ideal gas, internal energy 34 42 157
Ideal gas, specific heat 161
Ideal paramagnetic substance 15
Ideal solid solution 201
Ideal solution 201 231 256 257
Independent variables (quantities) 3 16 133 139
Infinitesimal process 4
Integrating denominator 71
Integrating factor 117
Intensive quantity 3
Internal energy 5 136 147
Internal force 10
Internal friction 37
Internal variable 3 195
Inversion temperature (point) of the Joule — Thomson effect 94 124
Ionic strength 204
Ionization energy 226
Iron ammonium alum 112
Irreversible cycle 61 62
Irreversible engine 62
Irreversible process 62
Isolated system 1
Isotherm 210
Isothermal bulk modulus 30
Isothermal compressibility 23 88 93 103 161
Isothermal magnetic susceptibility 35 89 93 104 163 164
Isothermal process, quasi-static 4
Jacobian 139
Joule — Thomson coefficient 91 94 126
Joule — Thomson effect 94 126
Joule — Thomson porous plug experiment 28 91
Joule — Thomson process 161 170
Joule's cycle 85
Joule's effect 166 188
Joule's effect of 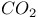 109 109
Joule's heat 59
Kalorische Zustandsgleichung 147
Kelvin temperature 11 65
Kelvin's equation 94
Kramers function 136
Laplace's formula 229
Latent heat of expansion 93 121
Latent heat of vaporization 247
Law of conservation of energy 6
Law of mass action 206
Le Chatelier — Brown's principle 145 161 172 180 242
Le Chatelier's principle 145
Legendre transformation 137
Liquefaction 170
Liquid 199
Liquid phase 210 214
Liquidous line 239 258 274
Local equilibrium 2 74
Macroscopic state 1
Magnetic body 59
Magnetic dipole 46
Magnetic field 15 34 35 47 164
Magnetic induction 18 58
magnetic moment 34
Magnetic refrigirator 113
Magnetic substance 15
magnetization 15 34 46 59
Magnetostriction 93
Many-component solution 200
Many-component system 193
Masieu function 136
Mass action 7 9 140 193 209
Maximum work 77
Maxwell's equation 37 57
Maxwell's Relation 89 106 107 109 139 155 169 179 181 188 269 286
Maxwell's rule 28 212 228 242 244 284
Mayer's cycle 33
Mayer's relation 21 49 120
Mechanical action 140
Mechanical equilibrium 140
Mechanical equivalent of heat 7
Mechanical interaction 2
Meissner effect 238
Melting 216 257
Melting point 209
Mercury 235
Metastable equilibrium 142 247
Minimum work 77 84 94 114
Mol fraction 198 202
Molality 202
Molar heat capacity 11
Molar specific heat at constant volume 11
Mole number 9
Molecule 197
Most stable equilibrium state 142
Natural variables 133 136
Negative specific heat 269 271
Nickel 288
Non-holonomic system 92
Non-volatile solute 256
Normal state 20
Occlusion 234
Ohm's law 59
Open system 1
Osmotic coefficient 224
Osmotic pressure 218 219 280
Otto's cycle 85
Oxidation 207
Oxygen 31 33 38
Paramagnetic salt 113
Paramagnetic substance, entropy 122 163 182
Paramagnetic substance, free energy 163 165
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



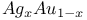
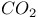 , Joule — Thomson coefficient
, Joule — Thomson coefficient 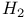 , enthalpy
, enthalpy 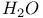
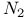
 -phase
-phase  -phase
-phase