Авторизация
Поиск по указателям
Prigogine I. — Advances in Chemical Physics, Vol. 2
Обсудите книгу на научном форуме Нашли опечатку?
Название: Advances in Chemical Physics, Vol. 2Автор: Prigogine I. Аннотация: In the last decades, Chemical Physics has attracted an ever increasing amount of interest. The variety of problems, such as those of chemical kinetics, molecular physics, molecular spectros-copy, transport processes, thermodynamics, the study of the state of matter, and the variety of experimental methods used, makes the great development of this field understandable. But the consequence of this breadth of subject matter has been the scattering of the relevant literature in a great number of publications.
Язык: Рубрика: Физика /Статус предметного указателя: Готов указатель с номерами страниц ed2k: ed2k stats Год издания: 1959Количество страниц: 412Добавлена в каталог: 11.04.2010Операции: Положить на полку |
Скопировать ссылку для форума | Скопировать ID
Предметный указатель
Inoue, S. 157 (ref. 185 Intramolecular London energies 74 78 Iodine molecule intramolecular London energy 78 Iodoform, ionization potential 198 Iodoform, quadrupole resonance of 191 198 201 204 Iodoform, resonance line 193 Iodoform, sulfur (CHl 196 Iodoform, sulfur crystal (CHl 195 196 Iodoform, sulfur crystal (CHl 191 Iodomethane hydrate, thermodynamic data and lattice constants 8 Ionic polymerization 148 160 161 Ionization energy 66 67 69 81 Ionizing radiations 197 Iron atom, energy levels of 313 Iron-nickel, (Fe 133 Iron-nickel, solid solution 125 Iron-nickel, system ( 133 Isobutene polymerized 157 Isoelectronic system 246 Isomerization energies, for butane 75 Isomerization energies, for pentane 75 Isoprene 169 Isotactic placement 172 Itoh 258 310 370 378 (ref. 393 Itterbeek, A. van 109 116 Ivash, E. V. 370 Ivohnstamm, Ph. K. 23 (ref. 57 Ivotani 258 307 Jahns, W. 3 (ref. 8 54 (ref. 57 James, H. 241 251 297 299 300 304 322 Jansen, L. 60 82 Jehle, H. 60 83 Jenkins, A. D. 174 (ref. 185 Jepson, W. B. 98 99 (ref. 103 106 107 (ref. 113 116 Johansson, C. H. 126 (ref. 145 Johnson, R. D. 371 378 (ref. 393 Jones, F. W. 128 146 Jones, H. 120 145 Joos, G. 114 116 Jucys, A. 296 Kanarek, E. 191 (ref. 194-196 (ref. 201 (ref. 202 (ref. 205 Karle, I. L. 373 (ref. 393 Kato 299 Katz, D. L. 44 48 54 55-57 97 116 Keating, D. 129 (ref. 145 Keevil, N. B. 91 92 (ref. 116 Kemp, J. D. 368 (ref. 392 Kennedy, G. C. 99 116 Ketelaar, J. A. A. 75 76 (ref. 82 Kihara 73 82 Kilb, R. W. 371 378 (ref. 392 Kilpatrick, J. E. 371 Kincaid, J. F. 165 (ref. 166 169 (ref. 185 221 322 King 295 Kinoshita 251 252 257 295 297- 299 Kirkwood, J. G. 60 63 67 68 82 83 Kisliuk, P. 382 (ref. 392 Kistiakowsky, G. B. 368 (ref. 392 Kivelson, D. 371 381 392 Kleppa, O. J. 133 137 142 (ref. 145 Kluiver, II. de 114 116 Kojima 370 Kono, H. 128 145 Korotkov, A. A. 160 185 Korvezee, A. E. 56 Kovalskaya, M. P. 2 (ref. 56 Kr 188 (ref. 205 Kreevoy 390 (ref. 392 Krisement, O. 257 306 Krisher, L. C. 371 Krypton, clathrates 20 30 39 41 Krypton, hydrates 3 32 34 Krypton, hydrates, polarizability, magnetic susceptibility, and ionization energy 70 Krypton, hydrates, thermodynamic data and lattice constants 8 Krypton, polarizability, potential energy 71 Kubaschewski, O. 121 (refs. 25 26) 132 136 (ref. 142 (ref. 144 (ref. 145 L 64 (ref. 82 207-322 Ladacki 154 (ref. 177 (ref. 185 Langmuir, constants 48 Langmuir, isotherm 39 Langmuir, theorem 15 Lassettre, F. N. 389 (ref. 392 Lattice model 123 Laurie, V. W. 370 371 Laves, F. 120 121 145 Le F 80 82 Le F 80 82 Lead, excess entropy of solution of noble metals in 133 Lead-thalium, solid solution 126 Lead-tin, solution, enthalpy of formation 143 Lead-tin, system, energy of solution 143 Lead-zinc, alloy (Pb 136 Legendre expansion in total ground state wave function of helium 294 Lennard - Jones and Devonshire, method in analysis of thermodynamic quantities of clathrates 24 25 28 33 34 47 Lennard - Jones, J. E. 24 25 28 258 Lennard-Jones six-twelve potential, in analysis of thermodynamic quantities of metallic solutions 135 Letort 163 185 Levy 149 (ref. 154 (ref. 177 (ref. 186 Lewis, M. N. 244 (ref. 258 (ref. 301 (ref. 321 Li, Y. Y. 122 127 128 145 Liander, H. 91 92 117 Lidc, D. R., Jr 370 371 378 (ref. 392 Lin, C. C. 371 378 (ref. 392 Linde, J. O. 126 (ref. 145 Lipkin, D. 154 (ref. 186 Lipscomb, W. N. 73 82 Lipsky, S. 199 (ref. 205 Liquid gas boundary curves 87 Lithium atom, ground state of 313 Lithium hydride, electronic correlation 324 Lithium hydride, superposition of configuration 296 Lithium ion Li 299 Lithium ion Li 249 254 324 Lithium ion Li 302 312 Lithium molecular superposition of configurations 296 Living ends 178 Living polymers 147 177-179 181 184 188 Living polystyrene 179-182 Local energy 299 Localizability theory 258 323 London energies for helium and hydrogen molecules 61 London forces between two hydrogen atoms 60 61 64 67 68 81 282 London, F. 59 60 66 76 81 82 249 259 Long range order 128 Longuet - Higgins, H. C. 135 (ref. 145 Lonsdale, H. K. 110 111 116 Lurie, E. 98 104 (ref. 116 Lynn, G. 92 (ref. 118 M 68 82 M 3 4 (ref. 7-9 10 21 23 (ref. 41 (ref. 50 (ref. 57 M 283 Maass, O. 100 116 MacDonald, J. K. L. 265 (ref. 322 Magat 197 (ref. 205 Magee, J. L. 199 205 Magnesium cadmium, alloy (Mg 129 Magnesium cadmium, alloy (MgCd 128 Magnetic entropy 132 Magnetic entropy, disordering 131 Magnetic heat capacity of nickel 133 magnetic susceptibility 25 Maleic anhydride 168 Mann, D. E. 371 378 (ref. 392 Many electron system, correlations in 304 305 318 319 323 Margenau, H. 60 68 76 (ref. 82 Mark, H. F. 156 162 185 Marsh, R. E. 3 4 56 Martin, D. L. 128 (ref. 145 Marvel, C. S. 164 Maslen, V. W. 232 Mason, E. 110 111 116 390 392 Massalski, T. B. 121 145 Mathot, V. 130 (ref. 136 (ref. 141 146 Matlock, A. S. 181 (ref. 185 Matsen, F. A. 276 Matthes, H. 125 (ref. 132 (ref. 146 Mayer, J. E. 60 82 104 (ref. 116 320 391 (ref. 393 McCain, G. H. 165 (ref. 185 McCormick, H. W. 182 185 McCullough, J. P. 371 McGinnies, R. 60 82 McGlashan, M. L. 122 128 145 McHaffie, I. R. 98 104 (ref. 116 McManamey, W. J. 73 82 McWeeny, R. 227 Meckler, A. 276 Medvedev, S. 151 (ref. 185 Meinhold, W. 3 (ref. 5 (ref. 10 18 32 (ref. 34 44 52 57 Melting temperature and critical temperature for disordering correlation 129 Melville, H. W. 163 186 Metallic solutions 120 Methacrylonitrile 155 Methane, 97 Methane, hydrate 33 34 47 Methane, hydrate, thermodynamic data and lattice energy 8 Methane, hydrogen sulfide-water ternary system 44 Methane, in clathrates 30 41 Methane, Langmuir constants 47 Methane, molecule, ionization energy 71 Methane, molecule, magnetic susceptibility 70 71 Methane, molecule, polarizability 71 Methane, propane-water ternary system 10 33 46 48 Methane, thiol hydrate 10 Methane, thiol hydrate, thermodynamic data and lattice constant 8 Methanol clathrates 2 20 Methanol clathrates, in hydroquinone 7 Methyl alcohol, barrier force of internal rotation 381 Methyl alcohol, microwave spectroscopy 379 Methyl boron difluoride 382 Methyl cyanide, clathrate of 20 Methyl ether 99 Methyl fluorosilane, barrier height of internal rotation 383 Methyl germane, barrier height of internal rotation 382 383 Methyl germane, microwave spectroscopy 380 Methyl methacrylate 155 Methyl methacrylate, polymerization 180 Methyl silane, barrier of internal rotation 381 382 383 391 Methyl silane, microwave spectroscopy 380 Meyer, G. 4 9 42 43 56 Meyer, H. 25 (ref. 56 Michel, R. E. 190 197 (ref. 205 Michels, A. 107 108 110 111 114 116 Microwave, methods 368 377 380 Microwave, spectroscopy 368 376 383 Midtdal, J. 257 298 Migirdicyan, E. 197 (ref. 205 Milkovitch, R. 149 (ref. 154 177 43) 185 186 Miller, B. 9 Miller, J. G. 110 111 116 117 Mixed crystals, quadrupolar resonance intensity in 190 Mixed hydrate 10 11 52 Mizushima, S. 372 (ref. 389 392 Moesta, H. 108 110 111 70) 112 117 Moffit, W. 259 Molar heat functions of clathrates 21 Molar volumes of clathrates 21 Monfils, A. 190 191 192 (ref. 193 205 Monodispersed polymers 177 Monodispersed systems 183 Moore, C. 240 322 Morino, Y. 373 (ref. 382 (ref. 393 Muldawer, L. 129 (ref. 145 Mulder 244 (ref. 258 (ref. 301 (ref. 321 Mulders, E. M. J. 9 10 22 43 56 Mulliken, R. S. 78 82 258 307 Munschy, G. 298 Munster, A. 144 Murphy, W. K 126 (ref. 127 134 138 (ref. 139 (ref. 142 46 47 49) 144 145 146 Myers, R. J. 371 378 (ref. 393 Nagasaki, S. 128 145 Nanassy, A. J. 114 Naphthalene, complex with sodium 153 Naphthalene, polymerization 150 Naphthalene, resonance line 191 Natta, G. 156 166 186 Naylor, R. E., Jr 371 Neon, atom in clathrates 12 20 Neon, intramolecular six-twelve potential energy 71 Neon, polarizability, magnetic susceptibility, and ionization energy 71 Nesbet, R. K. 283 319 Neumann, J. v 286 (ref. 322 Newkirk, J. B. 128 (refs. 34 35) 145 Newman, M. S. 368 (ref. 393 Newton - Raphson procedure 272 273 Nickel-gold, solid solution (Au 144 Nickel-gold, solution (Au - Ni), enthalpy of formation 143 Nickel-gold, solution (Au - Ni), short range order in 144 Nickel-iron system (Fe - Ni), small deviations from ideality 133 Nickel-platinum alloy 128 Nieuwenberg, C. J. van 99 117 Nikitin, B. A. 2 (refs. 20 21) 3 44 56 Nishikawa 370 378 (ref. 393 Nitrogen, 98 Nitrogen, 99 Nitrogen, 96 112 Nitrogen, hydrate 32 Nitrogen, in clathrate 30 Nitrogen, molecule, clathrate 2 Nitrogen, molecule, magnetic susceptibility of 70 Nitrogen, molecule, polarizability, magnetic susceptibility and ionization energy 71 Nitrogen, oxide hydrate, thermodynamic and lattice constants 8 Nitrogen, polarizability, potential energy 71 Nitromethane, barrier height of internal rotation 382 Nitromethane, microwave spectroscopy 379 Noaker, L. J. 44 56 Noble gas atoms polarizability 64 Nuclear magnetic resistance 194 Nuclear magnetic resonance 34 375 Nuclear quadrupole resonance 188 O'Brien 25 (ref. 34 56 O'Dnscoll, K. F. 150 (refs. 29a 29b) 186 Oite, K. 163 (ref. 185 One particle scheme 210 One-electron function 228 261 Oosterhoff, E. 389 (ref. 393 Ordered configuration 261 Oriani, R. A. 119-146 Ortho-iodo-phenol, radiation resistance 196 Osborne, D. W. 370 Osgan 168 (ref. 186 Overlap matrix 229 Oxygen, in clathrates 6 30 Oxygen, in hydrate 32 Oxygen, molecule clathrates 2 Palin, D. E. 2 6 7 (ref. 20 (ref. 56 Para-dibromo-diphenyl, radiation resistance 196 Para-dibromobenzene, radiation resistance 196 Para-dibromophenyl, radiation resistance 196 Para-dichlorobenzene, radiation resistance 196 Para-diiodobenzene, radiation resistance 196 Para-iodophenol, radiation resistance 196 Pariser, R. 259 Parr, R. G. 258 259 276 307 310 Patat, F. 156 186 Paul, D. E. 154 (ref. 186 Pauling, L. 3 4 9 25 (ref. 29 31 61 82 275 (ref. 282 (ref. 322 387 388 390 393 Pearse, J. F. 73 82 Pearson, D 114 (ref. 115 Pekeris, C. L. 252 Pennington, R. E. 371
Реклама
 |
|
О проекте
|
|
О проекте



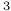 3 S
3 S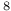 ) radiation resistance
) radiation resistance 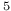 Ni
Ni - Fe - Ni) small deviation from ideality
- Fe - Ni) small deviation from ideality 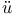 ger, H
ger, H  wdin, P. - O.
wdin, P. - O.  vre, C. G.
vre, C. G.  Zn
Zn ), calculation of thermodynamic quantities
), calculation of thermodynamic quantities 
 ller, Chr
ller, Chr  carbon dioxide system
carbon dioxide system 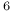 Ni
Ni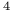 ) enthalpy of formation
) enthalpy of formation