Авторизация
Поиск по указателям
Prigogine I. — Advances in Chemical Physics, Vol. 2
Обсудите книгу на научном форуме Нашли опечатку?
Название: Advances in Chemical Physics, Vol. 2Автор: Prigogine I. Аннотация: In the last decades, Chemical Physics has attracted an ever increasing amount of interest. The variety of problems, such as those of chemical kinetics, molecular physics, molecular spectros-copy, transport processes, thermodynamics, the study of the state of matter, and the variety of experimental methods used, makes the great development of this field understandable. But the consequence of this breadth of subject matter has been the scattering of the relevant literature in a great number of publications.
Язык: Рубрика: Физика /Статус предметного указателя: Готов указатель с номерами страниц ed2k: ed2k stats Год издания: 1959Количество страниц: 412Добавлена в каталог: 11.04.2010Операции: Положить на полку |
Скопировать ссылку для форума | Скопировать ID
Предметный указатель
Diepen, G. A. 8 41 (ref. 55 100 31) 101 (ref. 102 31) 103 (ref. 115 116 Difluoroethane, hydrate 55 Difluoroethane, water-hydrogen sulfide ternary system 55 Diiodobenzene quadrupole spectrum 197 Diisocyanates, polymerization of 163 Dingemans, P. 90 (ref. 91 (ref. 116 Dinkelacker, O. 114 116 Dioxide hydrate 43 Dipole-dipole interaction 60 Dirac, P. A. 229 321 Dispersion of sound measurements of barriers of internal rotation 376 Dissociation pressures 33 Dixon, W. 371 Dode 129 144 Dokoupil, Z. 96 106-109 116 Donath, W. E. 61 63 64 66 69 76 82 Doninck, W. van 109 116 Donnelly, H. G. 97 116 Double hydrates 52 Dow Chemical Co 168 185 Dreyfus, B. 191 (refs. 14 15) 205 Duchesne, J. 187-206 Dunn, C. G. 297 Duwez, P. 121 144 Eckart, C. 263 307 321 Eckart, criteria 264 298 Eckart, procedure 267 Edwards, D. A. 128 (ref. 144 Effective charge 274 276 Effective Hamiltonian 226 Eirich, F. 156 185 Eisenschitz, R. 82 Elastic model, excess entropy calculation from 141 Elastic model, of a solid solution 140 Elbert, D. 297 Electric correlation 248 Electric field gradient 188 189 Electron(s) 200 Electron(s), correlation energy 74 Electron(s), diffraction 372 Electron(s), paramagnetic resonance 34 199 Electron(s), transfer and polymerization 154 Electron(s), transfer process and polymerization 150 181 Electronic specific heat of a brasses 131 Electrostatic interaction 391 Energy, matrix 271 Energy, of binding 34 Entropy, and hindered rotation 369 Entropy, influence of internal rotation 368 Entropy, of mixing 130 Entropy, of solid solutions 130 Entropy, of the clathrate 25 Epstein, P. S. 275 (ref. 321 Equilibrium clathrate 18 Equilibrium constants, influence of internal rotation on 368 Esch, U. 128 (ref. 144 Ethane, 101 Ethane, 100 101 Ethane, 101 Ethane, 101 Ethane, 100 Ethane, barrier of internal rotation 382 384 391 Ethane, clathrates 2 Ethane, hydrate thermodynamic data and lattice constants 43 Ethane, hydrates of 32 33 34 Ethane, infrared spectroscopy 373 Ethyl chloride, barrier height of internal rotation 383 Ethyl chloride, microwave spectroscopy of 380 Ethyl ether, 94 Ethyl ether, 94 ethylene 102 Ethylene, 102 103 Ethylene, clathrate 41 Ethylene, hydrate 32 33 Ethylene, hydrate, thermodynamic data and lattice constants 8 Ethylene, oxide 155 Eucken, A. 370 Eutectic line 88 Eutectic point 87 Evans, A. G. 162 185 Evans, D. F. 12 (ref. 21 33 56 Evans, E. Ll. 121 (ref. 145 Ewald, A. H. 95 96 99 103 (ref. 106 107 (ref. 110 112 (ref. 116 Excess entropy 130 131 Excess entropy, of solution 133 Excess entropy, of solution, calculation from elastic model 141 Excess entropy, of solution, of metallic solution 133 Excess free energy of solution 135 Exchange energy 209 Exchange integrals 384 385 Exchange polarization 313 Exchange polarization, excitation 199 Excited states 263 Eyring, H. 384 386-388 392 Fano, U. 199 205 Fermi hole 141 217 218 232 247 ferromagnetism 313 Field, T. E. 98 116 Fiirchtbauer, C. 114 116 Fischer 1 245 (ref. 321 Flinn, P. S. 126 (ref. 144 (ref. 144 Floating point 276 Flory, P. J. 164 185 Fluorine, ion, electronic correlation energy 240 Fluorine, ion, London energy 78 Fluorine, ion, molecules, intramolecular London energies 78 Fluoroethane barrier value of internal rotation 378 Fock - Dirac density matrix 225- Fock, V. 219 231 298 321 Forcrand, R. dc 3 43 56 Fordham, J. L. 157 165 (ref. 185 Fowler, R. H. 24 (ref. 25 (ref. 28 (ref. 56 Fox, T. G. 165 (ref. 166 (ref. 169 (ref. 185 Fr 239 240 Framework 379 Francis, A. W. 103 116 Franck - Condon principle 199 Franck, E. U. 78 (ref. 82 106 370 Free volume 26 27 33 Freeman, A. J. 238 321 Freeman, P. I. 103 (ref. 116 Friedel, J. 139 (ref. 144 Friedlander, H. N. 163 185 Friihbuss, H. 3 (ref. 49 52 57 Frisch, H. L. 165 168 185 Frost, A. A. 303 304 Frost, E. 9 Fuzukawa, J. 157 (ref. 185 Gamma hexachlorobenzene, radiation resistance 196 Gamma hexachlorocyclohexane, radiation resistance 196 Gamma-rays 188 193 194 197 202 Gamma-space 320 Gas hydrates 3 20 22 25 34 Gas hydrates, mechanism of formation 4 Gas hydrates, thermodynamic properties 15 Gas hydrates, x-ray work 3 Gaussian elimination technique 291 Gaussian wave function 276 Gegen ions 160 Geisler, A. H. 128 (ref. 145 Geometric mean approximation 29 Gerhauser, J. 276 Germane barrier of internal rotation 391 Giauque, W. F. 370 Gibbons, J. J., Jr 297 Gibbs - Duhem equation 16 Gibbs, free energy 30 Gibbs, function 20 Gillespie, L. J. 98 104 (ref. 116 Gitter, F. L. 370 Goff, J. A. 111 117 Gold, partial molar heat on solution in tin 133 Gold-copper, alloy (Au 136 142 Gold-copper, solid solution (CuAu) 129 Gold-lead, alloy (Au 136 Gold-nickel, alloy (Au 136 142 Gold-nickel, enthalpy of formation 143 Gold-nickel, solid solution (Au 144 Gold-nickel, solution, short range order in 144 Gold-silver, alloy (Au 136 142 Gold-thallium, alloy (Au 136 Gold-tin, alloy (Au 136 Goode, W. E. 165 (ref. 166 169 (ref. 185 Gordon, J. 370 Gorin, E. 386 (ref. 392 Gr 298 Graham, D. 376 (ref. 392 Gratch, S. 97 111 116 165 (ref. 166 (ref. 169 (ref. 185 Green, L. C. 235 236 239 244 (ref. 252 258 (ref. 301 (ref. 312 321 Grosjean, D. 190 191 193 205 Ground state, energy calculation 263 Ground state, treatment 261 Guggenheim, E. A. 23 (ref. 24 (ref. 25 (ref. 28 (ref. 56 120 122 (ref. 144 Gunst, C. A. van 100 (ref. 102 103 (ref. 116 Gutowsky, H. S. 191 204 206 376 (ref. 383 (ref. 392 Guttman, L. 124 125 127 128 129 (ref. 144 Gwinn, W. D. 371 378 (ref. 393 Hadni, A. 374 (ref. 392 Halford, J. O. 370 Hamann, S. D. 73 82 Hamilton, R. A. 110 (refs. 11 12) 111 115 Hamiltonian operator, Bethe - Salpeter's 240 Hamiltonian operator, separated in zero, one, two electron terms 212 Hammond, V. J. 76 82 Hard sphere approximation in calculation of thermodynamic quantities of clathrates 29 30 Harper, R. C. 110 116 Hartree - Fock, approximation 210 229 232 234 235 241 246 247 261 296 306 324 Hartree - Fock, energy 225 233 237 238 239 245 254 284 309 310 Hartree - Fock, function 226 227 234 240 242 253 280 305 Hartree - Fock, function orbital for hydrogen molecule 243 Hartree - Fock, magnetic properties 248 Hartree - Fock, scheme 223 224 231 234 242 245 248 258 278 293 307 309 311 313-315 317 319-321 Hartree - Fock, scheme, for helium 316 Hartree, D. R. 226 231 322 Hartree, scheme 232 247 Hartree, scheme, wave functions 63 236 Hartree, W. 231 322 Hass 302 Haugh, E. F. 76 77 82 Heat capacity of Au 132 Heat capacity of Au 369 Heat function for hydrates 33 Hecht 381 (ref. 392 Heitler, W. 249 259 Helium, 96 110 Helium, 95 112 Helium, atom in clathrate 12 Helium, carbon oxide intramolecular energy 110 Helium, electronic correlation 149 248 250 307 324 Helium, ground state energy of 252 295 298 301 302 307 311 Helium, Hartree - Fock energy 281 301 Helium, occupation numbers 280 Helium, polarizability, magnetic susceptibility and ionization energy 71 Helium, polarizability, potential energy 71 Helium-like ions, electronic correlation 239 240 300 303 324 Helium-like ions, ground state energy of 299 302 312 Hellman - Feynman equation 270 Herreilers, H. W. 9 Herschbach, D. R. 370 371 380 (ref. 392 Herzberg, G. 297 Heston, B. O. 9 55 Heterogeneous equilibria in clathrate solutions 34 35 Heterogeneous polymerization 163 Hexachlorobenzene, influence of radiation on 198 200 Hexachlorobenzene, quadrupole spectrum 197 Hexachlorocyclohexane resonance lines 191 Hexachlorocyclohexane resonance lines, quadrupole spectrum 195 197 Hexachlorodisilane barrier height of internal rotation 382 Higginson, W. 177 (ref. 185 Hilfsgas 18 Hill, A. E. 92 (ref. 118 Hindered rotation 33 34 Hindered rotation, internal 367 Hirabayashi 124 (ref. 128 129 16) 144 145 Hirota, E. 373 (ref. 382 (ref. 393 Hirschfelder, J. O. 28 (ref. 29 57 60 70 71 76 77 82 106 (ref. 108 116 221 275 281 322 Hoffman, j. D. 128 (ref. 146 Hofstadter, R. 200 (ref. 205 Hol 275 Holder, C. H. 100 116 Holm, C. H. 376 (ref. 383 (ref. 392 Homopolymer 168 183 Hossenlopp, I. A. 371 Hot bands 374 Hot lattice 4 11 21 Howard, J. B. 368 (ref. 392 Howe, L. S. 8 41 (ref. 56 Hoytink, G. 155 (ref. 185 Huggett 165) (ref. 166 169 (ref. 185 Hughes, R. E. 168 (ref. 186 Hulse, G. E. 181 (ref. 185 Hume - Rothery, W. 120 145 Hurley, A. C. 258 Hydrates 7 9 21 31 41 Hydrates, crystallization 44 Hydrochloric acid clathrates 2 Hydrochloric acid clathrates, in hydroquinone 7 Hydrogen molecule, carbon oxide intramolecular energy 110 Hydrogen molecule, clathrates 12 20 Hydrogen molecule, correlated wave function 300 Hydrogen molecule, correlation effects in 250 Hydrogen molecule, electronic correlation energy 241 245 248 258 Hydrogen molecule, electronic energy of 300 Hydrogen molecule, electronic wave function of 303 Hydrogen molecule, ground state energy of 300 307 Hydrogen molecule, magnetic susceptibility of 70 Hydrogen molecule, polarizability, magnetic susceptibility and ionization energy 71 Hydrogen molecule, polarizability, potential energy 71 Hydrogen molecule, wave function 243 Hydrogen negative ion H 299 Hydrogen negative ion H 240 Hydrogen negative ion H 249 324 Hydrogen negative ion H 302 303 312 Hydrogen, 110 Hydrogen, 96 108 Hydrogen, 108 Hydrogen, 96 112 Hydrogen, bound 4 175 Hydrogen, bromine hydrate 35 Hydrogen, chloride hydrate 35 Hydrogen, clathrates 2 Hydrogen, clathrates, chloride 30 Hydrogen, sulfide hydrate 3 42 50 Hydrogen, sulfide, as “hilfsgas” 18 Hydrogen, thermodynamic data and lattice constants 8 Hydrogen-like, function 274 318 Hydrogen-like, orbitals 274 275 Hydroquinone, cage 28 Hydroquinone, clathrate 2 5 6 11 20 21 29 35 39 43 Hydroquinone, clathrate, argon 38 41 Hydroquinone, clathrate, energies of formation 30 Hydroquinone, clathrate, equilibrium pressure 30 Hydroquinone, clathrate, methanol 39-41 Hydroquinone, clathrate, thermodynamic properties 5 Hydroquinone, rare gas system 35 Hylleraas, E. A. 211 219 221 239 249-251 256 257 265 (ref. 275 294 297 298 301 307 322 Ideal localized adsorption 5 10 Idelson 171 (ref. 185 Infrared method 373 374 Initiators of polymerization 148
Реклама
 |
|
О проекте
|
|
О проекте



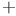
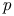 -bromochlorobenzene system
-bromochlorobenzene system  man, A.
man, A. 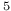 Cu
Cu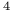 ), enthalpy of formation
), enthalpy of formation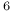 Ag
Ag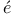 , H. R.
, H. R.  Ni
Ni solid solution
solid solution  ien, E.
ien, E. 