|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Vincenti W.G., Kruger C.H. — Introduction to Physical Gas Dynamics |
|
|
 |
| Предметный указатель |
Absolute reaction rates, theory of 216
Absorption 267
Absorption coefficient 444 446 457 460 463
Absorption coefficient, grey 470
Absorption coefficient, Planck mean 466
Absorption coefficient, Rosseland mean 469
Absorption lines 453
Absorption of radiation 455 461
Absorptivity, radiant 451
Accommodation coefficient 426
Acoustic equation, for radiative flow 496 497
Acoustic equation, for radiative flow for reactive flow 257
Acoustic theory, for radiative nonequilibrium 485 496
Acoustic theory, for reactive nonequilibrium 254
Acoustic wave, discrete 267 503
Acoustic wave, harmonic 262 499
Activation energy 214 223
Activation factor 215 221
Affinity 77
Air, chemical kinetics of 230
Air, chemical rate data for 231
Air, equilibrium constants for 168
Air, equilibrium properties at high temperatures 165 171
Air, physical constants for constituents of 524
Air, vibrational rate data for constituents of 205
Anharmonic correction, to internal energy 138
Anharmonic correction, to specific heat 138
Anharmonic effects in molecular vibration 137
Apse line 351
ApsevccLor 351
Arrhenius activation energy 214
Arrhenius equation 214
Assumption that 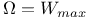 , assessment of 110 , assessment of 110
Atom-conservation equations 141 167
Average speed 47
Average value 31
Avogadro’s number 7 24 524
B — G — K model 376 422
Backward reaction 224
Billiard-ball molecular model 4
Bimodal distribution function 418
Bimolecular collision rate 52
Bimolecular mechanism 213
Black body 450
Boltzmann constant 7 524
Boltzmann distribution 108
Boltzmann distribution, physical meaning of 106
Boltzmann equation 328 332 369
Boltzmann limit 105
Boltzmann limit, condition for validity of 105
Boltzmann number 490
Boltzmann statistics 105
Boltzmann’s relation 115
Bose — Einstein statistics 95 98 447
Bouguer number 490
Bound-bound transitions 453
Bound-free transitions 453
Boundary conditions for flow with translational nonequilibrium 426
Boundary conditions for radiative flow 495 499 504 508
Boundary conditions for reactive flow 250
Bremsstrahlung 454
Bulk viscosity 407
Bulk-viscosity coefficient 391 410 411
Burnett equations 385 416
Caloric equation of state (see also Perfect gas Liquation
Caloric equation of state for complex gus mixture 170 523 526
Caloric equation of state for ideal dissociating gas 159
Caloric equation of state for symmetrical diatomic gas 154
Calorically perfect gas 8
Catalytic molecule 211
Center-of-mass reference frame 349
Chapman-Enskog distribution function 405
Chapman-Enskog expansion, Boltzmann equation 385
Chapman-Enskog expansion, Krook equation 379
Characteristic density for dissociation 158
Characteristic directions 272 302 303
Characteristic directions in linearized radiative flow 508
Characteristic temperature, for dissociation 153 524
Characteristic temperature, for electronic excitation 130
Characteristic temperature, for ionization 163 524
Characteristic temperature, for rotation 133 524
Characteristic temperature, for vibration 135 524
Characteristic time of rate process 236
Characteristics method of 300
Characteristics method of, theory of 273
Chemical equation 55 63 210
Chemical equilibrium 60
Chemical equilibrium by chemical thermodynamics 76 82
Chemical equilibrium by kinetic theory 55
Chemical equilibrium by statistical mechanics 139 148
Chemical equilibrium of complex mixtures 166
Chemical equilibrium, general equation for 76
Chemical kinetics 210 213
Chemical nonequilibrium 61 210
Chemical nonequilibrium, flow with 245
chemical potential 74
Chemical potential for thermally perfect gas 81
Chemical potential of translation 124
Chemical rate equation, general considerations 210 (see also Rate equation)
Chemical reaction, mechanism of 211 213
Chemical reactions, multiple simultaneous 65
Chemical thermodynamics 59
Chemical thermodynamics, alternative approaches to 59
Classical mechanics, use in statistical mechanics 89
Closed system 60
Cold-gas limit 498
Collision broadening 453
Collision frequency 13 379
Collision frequency in B — G — K model 379 384
Collision integral 41 354 361 370
Collision integral in Boltzmann equation 330
Collision integral, moments of 335 347 362 364
Collision rate 48
Collision theory of chemical reaction rates 210 216
Collision-dominated flow 380
Collisional invariants 348
Collisions, molecular 12 35 36 48 205 216 333 348
Collisions, molecular, small-deflection 360
Compatibility relations 302 303
Compressibility factor 154 172
Concave corner, linearized theory for 307
Concave corner, nonequilibrium flow over 306
Concave corner, nonlinear theory for 307
Concentration 56 211
Conservation equations, for chemically reacting gas mixture 372
Conservation equations, for equilibrium flow 345 380 386
Conservation equations, for flow through shock waves 179 516
Conservation equations, for flow with radiative nonequilibrium 474
Conservation equations, for flow with translational nonequilibrium 327 371 392 410
Conservation equations, for flow with vibrational or chemical nonequilibrium 246
Conservation equations, for radiative flow through normal shock wave 516
Conservation equations, for steady quasi-one-dimensional equilibrium flow 183
Conservation equations, for steady quasi-one-dimensional non-equilibrium flow 286
Conservation equations, in local natural coordinates 301
Conservation of energy 65
Conservation of mass in chemically reacting system 63
Continuity equation (see also Conservation equations)
Continuity equation for chemical species 249 372
Continuity equation, B — G — K solution 431
Continuity equation, free-molecule solution 428
Continuity equation, Lees solution 429
Continuity equation, Navier — Stokes solution 425 428
Continuum radiation 454
Convex corner, nonequilibrium flow over 310
Couette flow 424
Coupled reactions 286
Coupling between chemical reactions and vibration 228
Cross-section for absorption of radiation 445
Cross-section, collision 332 353 355 370
Cross-section, momentum-transfer 358
| Cross-section, Rutherford 361
Cross-section, total 357 360
Dalton’s law of partial pressures 9
Data, for constituents of air 135 205 230 524
Data, thermochemical 171
De Broglie relation 93
de Broglie wavelength 93 359
Deflection angle 351
Degeneracy of quantum energy level 110
Degenerate gas 105
Degenerate quantum states 110
Degree, of advancement of chemical reaction 63
Degree, of dissociation 152
Degree, of ionization 163
Depleting collision 36
Detailed balancing, principle of 42 166 200 224 456
Diatomic gas, equilibrium properties of 152
Diatomic gas, law of mass action for 153
Diatomic gas, rate equation for 232
Diatomic gas, statistical mechanics of 132
Differential approximation for radiative transfer 491 520
Diffraction effects 359
Diffuse reflection 426 481
Diffusion 15 250 372
Diffusion velocity 372 406
Diffusion, coefficient of 16 19 406
Diffusion, mutual 19
Diffusion, self 18
Direction of propagation of radiation 437
Discrete wave in radiating gas 503
Discrete wave in reacting gas 267
Disorder, molecular 113
dispersion 267
Dissociation energy 137 141
Dissociation reaction, kinetics of 222
Dissociation, characteristic density for 158
Dissociation, characteristic temperature for 153
Dissociation, degree of 152
Dissociation, effect on flow through normal shock wave 182
Dissociation-recombination reaction, kinetics of 226
Dissociation-recombination reaction, statistical treatment of 148
Distribution function 27 319
Distribution function as probability or expectation function 323 342
Distribution function for molecular speed 46
Distribution function for molecular velocity 44
Distribution function, Chapman — Enskog 405
Distribution function, Maxwellian 334
Distribution function, normalized 30 318
Distribution function, Photon 438
Doppler broadening 453
Drag, effect of nonequilibrium on 279 508
Eigenvalues 92
Einstein coefficients 454
Elastic collisions 348
Elastic reflection 5
Elastic-sphere molecules 4 356
Electron spin 163
Electronic excitation 130
Emission coefficient 444 457
Emission mean absorption coefficient 466
Emission-dominated approximation 477 494
Emission-dominated interaction 466
Energy density, molecular 319 324 326
Energy density, radiative 439 449
energy equation (see Conservation equations)
Energy flux, radiant 439
Energy flux, translational 320 324
energy levels 110
Energy levels of rigid rotator 132
Energy of dissociation 137 141
Energy states in quantum mechanics 89
Energy states of harmonic oscillator 134
Energy states of particle in rectangular box 92
Energy transport 18 325 326 391 406
Energy zero 156
Energy, common zero in reacting system 141
Ensemble of systems 120
Enthalpy 66 325 326 528
entropy 66 113 338
Entropy and disorder 113
Entropy of an isolated system 69
Entropy of electronic excitation 139
Entropy of mixing 80
Entropy of reacting system 145
Entropy of rotation 138
Entropy of translation 123
Entropy of vibration 138
Entropy production 68
Entropy production by chemical nonequilibrium 75
Entropy production by vibrational nonequilibrium 206
Entropy, Calorimetric 124
Entropy, flow of 68
Entropy, statistical 124
Equation for small departures from uniform free stream 271 505
Equation of radiative transfer 443 460 463 470
Equation of radiative transfer, formal solution of 463
Equation of radiative transfer, moments of 491
Equation of state for system in chemical nonequilibrium 62
Equation of state for thermally perfect gas 7 31 122
Equation of state of reacting system 146
Equations of transfer 346 364 370
Equilibrium condition, fictitious local 237 241
Equilibrium constant 57
Equilibrium constant for constituents of air 168
Equilibrium constant, lelation to rate constants 225
Equilibrium flow 178 252 345
Equilibrium Mach line and Mach angle 273
Equilibrium speed of sound 258 259
Equilibrium, chemical 76
Equilibrium, condition for 41
Equilibrium, kinds of 60
Equilibrium, mechanical 60
Equilibrium, Radiative 446 456
Equilibrium, thermal 60
Equilibrium, thermodynamic 60
Equilibrium, translational 334
Equipartition of energy, principle of 11
Eucken’s relation 21 407
Euler’s equations 247
Exclusion principle 96
Expansion into a vacuum 112
Exponential approximation 483 520
Extensive property 70
Factorization property of partition function 127
Fermi — Dirac statistics 96 99
First law of thermodynamics 65
Flow velocity 1 319 369 373
Flow with chemical nonequilibrium 245
Flow with ionizational nonequilibrium 251
Flow with radiative nonequilibrium 473
Flow with translational nonequilibrium 375
Flow with vibrational nonequilibrium 245
Flux, molecular 33 320
Forces, external 322 330
Forces, intermolecular 2 21 333 348 408
Forces, surface 322 324
Forward reaction 222
Fowler and Darwin, method of 101
Free-free transitions 454
Free-molecule flow 428
Freezing, in nozzle flow 298
Frequency, of molecular collisions 48
Frequency, of radiation 437
Frozen flow 191 253
Frozen Mach line and Mach angle 273
Frozen speed of sound 258 259
Fully dispersed wave 292 520
Fully excited energy 133
Gas constant, for frozen gas 193
Gas constant, molecular 7
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



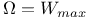 , assessment of
, assessment of