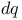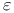|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Bryan G.H. — Thermodynamics: an introductory treatise |
|
|
 |
| Предметный указатель |
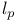 , , 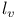 9 9
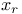 , , 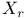 93 93
 9 112 9 112
 9 9
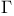 , , 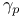 , , 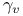 8 8
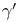 , , 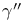 11 11
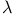 10 147 10 147
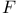 , , 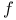 92 93 92 93
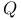 , , 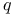 , , 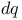 114 114
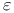 9 9
A 83 84 86 90
Absent constituents 158
Absolute temperature 18 54 127
Absolute unit of heat 14
Absolute zero 19
Accelerations-energy 191
Adiabatic transformation 5 22 49
Adiabatics, equation of, for a gas 119
Air thermometer 118
Amagat 139
Andrews 12
Areas, rule of equal 141
Availability 41
Available energy 35 42 83
Avariant system 146
B 117
Bancroft, Wilder D. 184
Battery, gas 166
Bivariant system 145
Black body radiations 99
Black body radiations, entropy of 103
Body, temperature of moving 66
Boltzmann — Maxwell law 189
Boltzmann’s investigation 101
Boyle 7 116
Burbury preface IV
Cagniard de la Tour 12
Calorie 4
Calorie, gram 4
Calorie, great 4
Calorie, kilogram 4
Calorie, small 4
Carnot 15 19
Carnot’s cycle 15 51
Carnot’s cycle, dynamical model of 185
Carnot’s function 19
Changes of phase 10
Charles’ or Gay Lussac’s law 117
Chemical changes and combustion 75
Clapeyron 15 19 109
Clausius 15 117 139 143 190
Clausius — Szily system 190
Clausius, inequalities 59 61
Coefficients of a gas 20 117
Coefficients of cubical expansion 9 112
Coefficients, differential 8 23
Coefficients, partial differential 23
Coefficients, thermal 7 20 108 111
Combustion and chemical changes 75
Compensating transformations 41
Complex, properties of a 177
Complex, saturated 10 140
Complex, supersaturated 140
Complex, thermodynamic model of 177 179
Complex, unsaturated 140
Compound system 5
Compressibility 9
Conduction of heat 69
Conservation of energy 31 43
Conservation of entropy 78
Conservation of heat 14
Constant pressure, specific heat at 8
Constant pressure, universal gas 118
Constant pressure, volume, specific heat at 8
Constants in the potentials 94
Constants of integration, arbitrary 40 66
Constituents, absent 158
Continuity of the liquid and gaseous states 178
Convection, electric 174
Conversion of work into heat, irreversible 71
Coordinates, force 30
Coordinates, generalised 93
Coordinates, partial potentials as 180
Coordinates, triangular 183
Critical phenomena 138
Critical point 12 137
Critical pressure 12
Critical temperature 12
Cubic expansion for a gas 20 117
Cubic expansion, coefficient of 9
Currents 162 etc.
Currents effects on localisation of entropy and energy 173
Curve of saturation 10 141
CYCLE 6
Cycle, Carnot’s 15 51
Cyclic transformation 6
Cyclic transformation, system 188
Davy 13
Degradation of energy 43
Density, maximum 12 24
Descartes 13
Determinants, functional 24
Diagrams, indicator 6
Diagrams, thetaphi 79
Differential coefficient 8
Differential condition for a perfect 108
Differential elements 37
Diffusion of gases 75 125 126
Directed radiation, entropy of 105
Dissipated energy, surface of 178
Duhem 95 193
Duhem theory of false equilibria 193
Dynamical and granular theories 30
Dynamical model of Carnot’s cycle 185
Dynamical system, rational 29
Dynamical unit of heat 14
d’Alembert’s principle 30
e, E 162
Effects, thermoelectric 164
Efficiency of a heat engine 17
Elastic solid 95
Elastic wire 111
Elasticity, modulus of 9 113
Electric convection 174
Electricity, flow along a wire of 73
Electricity, specific heat of 170
Electromotive force 170
Element, differential 37
Element, reversible galvanic 162
Energy 29
Energy in its most general aspect 38
Energy of complex 148
Energy, accelerations 191
Energy, available 35 42 83
Energy, conservation of 31 43
Energy, degradation of 43
Energy, determination of 132
Energy, effects of currents on 173
Energy, Helmholtz’s free 94
Energy, localisation of 31
Energy, mechanical 44
Energy, surface of dissipated 178
Energy, test of stability 80
Energy, unavailable 56 116
Engine, efficiency of a heat 17
entropy 57 69
Entropy and potentials, determination of 132
Entropy of a complex, whole 148
Entropy of a mixture 122
Entropy of black body radiation 103
| Entropy of directed radiation 105
Entropy, changes due to heat conduction 69
Entropy, conservation, localisation and transmission 78
Entropy, effects of the currents on localisation of energy and 173
Entropy, first definition 58 64
Entropy, gain of 125
Entropy, second definition 58 66 116
Entropy, temperature diagrams 79
Entropy, thermically heterogeneous system 68
Entropy, whole 68
Equal areas, rule of 141
Equation, fundamental 153 161
Equilibrium, Duhem’s theory of false 193
Equilibrium, phase 12 140 156
Equilibrium, stable 81 84
Equilibrium, temperature of 10
Equilibrium, thermal 49 50
Equivalence of heat and work 13
Equivalent, mechanical 14
Essentially stable states, metastable and 143
Expansion of a gas 20 117
Expansion, coefficient of cubic 9 112
Expansion, latent heat of 9 112
False equilibria, Duhem’s theory of 193
Faraday’s law 167
Ferguson preface VI
Final temperature 91
First definition of entropy 58 64
First law of thermodynamics 13 107
First scale, Kelvin’s 20
Flow of electricity along a wire 73
Fluid motion brought to rest by viscosity 73
Fluids, stability of homogeneous 84
Force coordinates 30
Force electromotive 170
Formation, heats of 164
Four thermodynamic relations, Maxwell’s 109
FOURIER 13
Freedom, mechanical 5
Freedom, unilateral 82
Friction 72
Function, Carnot’s 19
Functional determinants 24
Fundamental equation 153 161
G(p,T) 147
Galvanic element, reversible 162
Gas battery 166
Gas constant universal 118
Gas cubical expansion of a 117
Gas mixtures 121
Gas potential of 126 160
Gas rushing into a vacuum 73 120 131
Gas temperature 3
Gas thermometer 118 130
Gases, diffusion of 75 125 126
Gases, irreversible effects of mixing 124
Gases, perfect 116
Gay Lussac or Charles’ law 117
Geometrical interpretations 134
Gibbs 89 156 159 163 165 174 175
Gibbs formula for electromotive force 165
Gibbs stability conditions 89
Gibbs theory of chemical systems 151
Gilbault 167
Grain of entropy 125
Gram — Calorie 4
Granular medium 30
Granular theories 30
Great calorie 4
h, H, dh 114
Hamiltonian 29
Heat see “Latent heat” “Quantity “Specific “Unit etc.
Heating a body 4
Helmholtz 94 164 188
Helmholtz, “Free energy” 94
Heterogeneous systems, chemically 156
Heterogeneous systems, thermically 68
Hirn 14
Hoar-frost line 144
Homogeneous fluids, stability of 84
Homogeneous mixture 152
Homogeneous systems, thermically 49
Hooke 13
Ice line 144
Impact of imperfectly elastic bodies 73
Indicator diagrams 6
Inequalities, Clausius’ 59 61
Inner thermodynamic potential 95
Internal heat equilibrium 49
Intrinsic energy 32
Inversion of porous plug effect 131
Irreversibility 40
Irreversible conversion of work into heat 71
Irreversible processes 113
Irreversible radiation phenomena 106
Irreversible transformations 67
Isentropic coefficient of expansion 9
Isothermal transformations 5
j 14
Joule, James Prescott 13 14 117 128 138
K 8
Kamerlingh, Onnes Dr. 139
Kelvin, Lord 15 20 90 128 167 168
Kelvin’s expressions for available energy 90
Kelvin’s first scale 20
Kilogram calorie 4
Kinetic theory of gases 189
L 29
Latent heat of expansion 9 21 108 112
Latent heat of pressure-variation 9 21 108 112
Latent heat of transformation 10 11 147
Latent heat, relation with temperature 150
Leathern 38
Line, steam, ice, hoar-frost 144
Localisation of energy 31
Localisation of energy and entropy, effects of currents on 173
Localisation of entropy 78
Locke 13
Lummer 99
Lussac’s (Gay) law 117
Mariotte 7 116
Massieu, F. 93
Maximum density 12 26
Maxwell — Boltzmann distribution 189
Maxwell’s four thermodynamic relations 109
Mayer, Robert 13
Mechanical energy 44
Mechanical equivalent 14
Mechanical freedom 5
Medium, auxiliary 57
Medium, granular or newtonian 30
Mellor, J.W. 194
Metastable and essentially stable 143
Mixture, gas 121
Mixture, homogeneous 152
Mixture, partial potentials of the constituents of a 150
Mixture, potential of a 126 160
Mixture, whole entropy of 122
Model of Carnot’s cycle, dynamical 185
Model, thermodynamic 174
Modulus of elasticity 9 113
Modulus, Young’s 96
Molecular Volume 118
Monoclinic 146
Monocyclic system 188
Moutier’s rule 146
Moving body, temperature of 55
Mutual potential energy 32
Newtonian or granular medium 30
Non-conservation of heat 14
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



 ,
, 
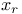 ,
, 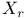


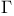 ,
, 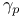 ,
, 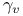
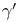 ,
, 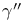
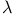
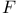 ,
, 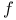
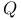 ,
, 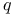 ,
,