|
|
 |
| јвторизаци€ |
|
|
 |
| ѕоиск по указател€м |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Adkins C.J. Ч Equilibrium Thermodynamics |
|
|
 |
| ѕредметный указатель |
 -Brass 82Ч83 -Brass 82Ч83
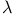 -points 197 -points 197
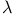 -transition 197 -transition 197
Absolute zero 23
Absolute zero, unattainability of 245Ч247
Absorptivity, spectral 147
Accessibility 88Ч92
Adiabatic changes 4
Adiabatic changes, uniqueness of reversible 60Ч61 72 92
Adiabatic demagnetization 134Ч136
Adiabatic demagnetization, cooling by 136Ч143
Adiabatic demagnetization, microscopic significance of 138
Adiabatic walls 4
Adiathermal changes 4
After-burners 48
Air standard cycles 65
Allotropic transformations 247Ч249
Arrow of time 74
Availability 107
Bernoulli's theorem 490
Binary mixtures 231Ч240
Binary mixtures, phase separation in 231Ч236
Binary mixtures, solid/liquid equilibrium 236Ч240
Black body 149
Black body radiation 148Ч149
Boltzmann relation 79Ч81
Boundaries 4
Boyle temperature 116
Boyle's law 116
Caloric theory 30
Caratheodory's principle 88
Carbon resistance thermometers 26
Carnot cycle 51Ч53
Carnot's theorem 56Ч58
Cells, electric 125Ч129
Cells, fuel 128
Celsius temperature 23
Centigrade temperature 23
Change of phase 180Ч212
Change of phase, Ehrenfest classification 193
Change of phase, first order 183
Change of phase, first order, Gibbs functions in 186Ч187
Change of phase, higher order 193Ч194
Change of phase, higher order, interpretation of 203Ч205
Charle's law 118
Chemical potentials 218Ч221
Chemical potentials in ideal gas mixtures 215 219 221
Chemical reactions 222Ч223
Chemical reactions in ideal gas mixtures 228Ч230
Clausius Ч Clapeyron equation 182Ч184
Clausius Ч Clapeyron equation, integration of 184Ч185
Clausius' theorem 68Ч71
Compliance coefficients 104
components 3
Components, systems of several 213Ч240
Compressibility, adiabatic 114Ч115
Compressibility, isothermal 37 115
Compression ratio 67
Condensation nuclei 211
Configuration 89
Conservation of energy 30 33
Constant-flow calorimeter 47Ч48
Corresponding states, law of 192 267
Cosmic black-body background 270
Countercurrent heat exchanger 169Ч170
Critical constants 122
Critical constants of Dieterici gas 266
Critical constants of van der Waals gas 267
Critical constants, table of 122
Critical magnetic field 200
Critical points 121 188Ч192
Critical temperature 121
Critical temperature of superconductors 200
Critical temperature table 122
Curie temperature 136Ч140
Curie Ч Weiss law 140
Curie's law 27 140
Curie's law, breakdown of 243
Curve differentials 43
Cyclic processes 50Ч53
Dalton's law 214
Daniel cell 125Ч126
Degradation of energy 76Ч77
Degrees of freedom 10Ч12 103 223Ч224
Demagnetizing factor 135
Diathermal walls 4
Dieterici equation 121
Dieterici equation, critical constants of 266
Dieterici equation, reduced form of 267
Differentials, exact 15
Differentials, inexact 16
Differentials, partial 13
Diffusion of ideal gases 216Ч218
Dilution refrigerator 236
Direct observables 3
Displacement law 156
Efficiency of heat engine 51
Efficiency of heat pump 63
Efficiency of refrigerator 62
Ehrenfest's equations 194
Elastic rod 124Ч125
Electric cells 125Ч129
Emissive power, spectral 147
Emissivity, spectral 149
Empirical entropy 88Ч92
Empirical entropy and heat 93
Empirical temperature 18Ч20
Endothermic reactions 128
Energy density of black body radiation 150Ч152
Energy density of radiation 145
Energy density, spectral 147
Energy density, spectral, of black body radiation 157
Energy of mixing 232
Enthalpy 45Ч46 100
entropy 68Ч81
Entropy and degradation of energy 76Ч77
Entropy and heat capacities 81Ч84
Entropy and order 77Ч81
Entropy in irreversible changes 72Ч74 95Ч97
Entropy of ideal gas 119Ч120
Entropy of ideal gas mixtures 215Ч216
Entropy of mixing 231
Entropy, a function of state 71Ч72 95
Entropy, definition of 71 95
Entropy, empirical 88Ч92
Entropy, empirical, and heat 93
Entropy, form of first law 74Ч76
Entropy, increase in diffusion 216Ч218
Entropy, law of increase of 74
Equal temperature enclosure 147
Equation of reaction equilibrium 223
Equation of state 20
Equation of state of a van der Waals gas 120
Equation of state of an ideal gas 117Ч118
Equation of state of Dieterici gas 121
Equilibrium 7Ч8
Equilibrium in mixtures 231Ч240
Equilibrium, constant 228 251Ч252
Equilibrium, fluctuations 157Ч161
Equilibrium, general conditions for 106Ч111 221Ч222
Equilibrium, heterogeneous 231
Equilibrium, metastable 7 187
Equilibrium, radiation 147Ч148
Equilibrium, types of 7Ч8
Equipartition 120
Equipartition and fluctuations 160
Eutectic composition 240
Eutectic temperature 240
Exact differentials 15
Exchanges, theory of 146
| Exothermic reactions 128
Expansion engine 172
Expansion ratio 67
Expansivity, cubic 37 114 131
Expansivity, linear 124
Extensive variables 5
ferromagnetism 83
Figure of merit of heat pumps 63
Figure of merit of refrigerators 62
First law 31
First law, differential form of 33Ч34
First law, entropy form of 74Ч76 96
First order phase change 183 193
First order phase change, Gibbs functions in 186Ч187
Flow processes 46Ч49
Flow work 47
Fluctuations 7 157Ч161
Fluctuations and equipartition 160
Fountain effect 205Ч208
Free energy 100 103
Free expansion 116 162Ч165
Frost 149Ч150
fuel cells 128
Full radiation 148
Functions of state 6
Gas constant 118
Gas thermometry 20Ч23 118
Gibbs function 100
Gibbs function in change of phase 180Ч182
Gibbs function in first order transitions 186Ч187
Gibbs function near critical points 188Ч192
Gibbs paradox 217Ч218
Gibbs Ч Helmholtz equation 101
Glass-houses 150
Glasses 249Ч251
Heat 32Ч33
heat capacity 42Ч45 81 112Ч114
Heat capacity anomalies 81Ч84
Heat capacity, specific 43
Heat content 46 100 127
Heat engines 51 56Ч58 64Ч65 97Ч98
Heat exchangers 169Ч170
Heat of reaction 46 127 229 251Ч252
Heat of solution 227
Heat pumps 62Ч63 98Ч99
Heat, caloric theory of 30
Heat, molecular motion theory of 30
Helium 192 196Ч197 205 244Ч245
Helium as refrigerant 137
Helium, fountain effect with 205Ч208
Helmholtz function 100
Henry's law 224
Heterogeneous equilibrium 231
Hotness 34
Hotness and temperature 20 55 97
Hysteresis 9Ч10
Ideal gas 116Ч120
Ideal gas mixtures 213Ч218
Ideal gas mixtures, entropy of 215Ч218
Ideal gas mixtures, Gibbs functions in 216
Ideal gas mixtures, partial potentials in 218
Ideal gas mixtures, pressure of 213Ч214
Ideal gas mixtures, reactions in 228Ч230
Ideal gas mixtures, reversible mixing of 214Ч215
Ideal gas, adiabatic equation of 118Ч119
Ideal gas, compression of 36Ч37
Ideal gas, definition of 116
Ideal gas, entropy of 119Ч120
Ideal gas, equation of state 117Ч118
Ideal gas, internal energy of 116Ч117
Ideal gas, temperature scale 20Ч21 118
Ideal solutions 224Ч228
Ideal solutions, solubility in 226Ч227
Ideal solutions, vapour pressure of 226
Indicator diagram 9
Inexact differentials 16
Intensive variables 5
Internal combustion engine 64Ч67
Internal energy 32
Internal pressure 121
Internal work 121
International Practical Temperature Scale 28Ч29
Inversion curve 167
Inversion temperature, maximum, table of 168
Iron 195
Irreversible changes 72Ч75 95Ч97 162Ч179
Isolation 4
Isotherms 18284
Jet engines 48
Joule coefficient 164
Joule cycle 263
Joule expansion 162Ч165
Joule Ч Kelvin coefficient 166
Joule Ч Kelvin expansion 165Ч168
Joule Ч Kelvin expansion and gas liquefaction 168Ч172
Joule Ч Thomson expansion 165
Joule's law 116 121
Kelvin 22
Kirchhoff's law 148Ч150
Knocking 67
Latent heat and order 85
Lattice temperature 142
Laws of thermodynamics 2Ч3
Laws of Thermodynamics, First 31
Laws of Thermodynamics, Second 53 88
Laws of Thermodynamics, Third 241 245 252
Laws of thermodynamics, zeroth 17
Legendre differential transformation 103Ч104
Lever rule 235
Liquefaction of gases 168Ч172
Liquid expansion thermometers 24
Liquidus 237
Magnetic energy 253Ч255
Magnetic work 40Ч42 253Ч255
Magnetocaloric effect 134Ч142
Mass action, law of 228Ч230
Maxwell relations 104Ч106
Meissner effect 198
Metastable equilibrium 7Ч8 186Ч187
Mixtures, binary 231Ч240
Mixtures, method of 34
Mixtures, solubility gaps in 231Ч236
Nernst statement of third law 252
Neutral equilibrium 7Ч8
Nozzle, ideal 48
Nuclear demagnetization 142
Nucleation 211Ч212
Order and entropy 77Ч81
Order change by deformation 86
Order, range of 205
Order-disorder transitions 81Ч84
Osmotic equilibrium 227
Osmotic pressure 227Ч228
Otto cycle 65Ч66
p-V-T relations 121Ч124
Paramagnetic salts 83
Paramagnetic salts, adiabatic demagnetization with 134Ч142
Partial differentials 13Ч15
Partial potential 218
Partial potential, molar 221
Partial pressure 214
Peltier effect 174
Perfect differentials 15
Perfect gas 116Ч120
Perfect gas mixtures 213Ч218
Perfect gas mixtures, entropy of 215Ч218
Perfect gas mixtures, Gibbs function in 216
Perfect gas mixtures, partial potentials in 218
Perfect gas mixtures, pressure of 213Ч214
Perfect gas mixtures, reactions in 228Ч230
|
|
 |
| –еклама |
 |
|
|
 |
|
ќ проекте
|
|
ќ проекте



 -Brass
-Brass 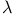 -points
-points