Авторизация
Поиск по указателям
Rao C>N> — Ultra-Violet and Visible Spectroscopy: Chemical Applications
Обсудите книгу на научном форуме Нашли опечатку?
Название: Ultra-Violet and Visible Spectroscopy: Chemical ApplicationsАвтор: Rao C>N> Аннотация: It would be superfluous to stress the importance of electronic .spectroscopy in structural or analytic research. It has now become a matter of routine to record the ultra-violet or visible spectra of compounds for purposes of identification or structure elucidation. The spectrophotonletric methods of analysis have replaced the conventional methods in ever so rnany instances. The number of-publications in the different branches of chemistry, reporting data on or making use of electronic spectroscopy, is ever-increasing and it would be impossible to review the field completely. In this book, I have tried to introduce the basic concepts of electronic spectroscopy and to present its applications in analytical, structural and physico-chemical problems. As far as possible, the rnateripl has been selected to illustrate the basic: principles of modern theories in chemistry. While surveying the data on organic molecules, several empirical generalizations have been discussed. lVlany of the more recent developments like tIle far ultra-violet spectra of organic molecules, fluorescence spectra, charge transfer spectra, ligand field theory, optical rotatory dispersion etc., have been briefly discussed in the latter part of the book. All through the discussion, the recent notations of electronic transitions have been used, although the other commonly encountered notations have also been mentioned. I shall be most gratified if this book can be of help to chemists in using electronic spectroscopy more effectively in research or routine work.
Язык: Рубрика: Физика /Статус предметного указателя: Готов указатель с номерами страниц ed2k: ed2k stats Издание: 1st editionГод издания: 1961Количество страниц: 164Добавлена в каталог: 30.08.2009Операции: Положить на полку |
Скопировать ссылку для форума | Скопировать ID
Предметный указатель
Cyanine dyes 35 89 134 Cyanine dyes, association 79 Cyclic compounds 101 Cyclo-octanone 19 Cyclo-octatetraene 49 Cyclobutadiene 49 Cyclobutanone 19 Cyclodecanone 19 Cyclohepta-1:3-diene 26 Cycloheptanone 19 Cycloheptatrienylium carbonium ion 49 Cyclohex-1-enylethylene 26 Cyclohexa-1:3-diene 25 26 Cyclohexanone 19 Cyclohexene 61 Cyclononanone 19 Cyclopentadiene 26 52 Cyclopentanone 18 19 Cyclopentanones, 29 Cyclopropane 15 90 Cyclopropyl conjugation 90 Cyclopropyl derivatives 90 Cyclopropyl group 41 Cyclopropyl ketones 90 Cyclopropyl-benzene 90 Decamethylbiphenyl 84 Depolarization 107 Di-aza-naphthalenes 54 Di-o-tolyl guanidine 104 Di-p-xylylene 89 Diacetyl 100 Diacetylenes 36 Diamagnetic solids 99 Dianisylmethylene anthrones 108 Dianthrone 109 Diaryl ketones 32 Dibiphenylene-butadiene 89 Dibiphenylene-ethylene 89 Dicarbonyl compounds 32 Dichroism, optical 121 Dichromates 143 Dienes 25 65 94 Dienes, cyclic 25 26 Dienes, heteroannular 67 Dienes, homoannular 67 Dienes, linear 25 26 Dienes, polycyclic 26 69 Dienes, semicyclic 25 26 Dienes, steroid 26 66 Dienes, triterpenoid 26 Dienones, 67 Diethyl ether 61 Diethyl keten 37 Diethylnitrosoamine 23 Difference spectrum 67 Dihydrojasmone 29 Diketones 89 Diketones cyclic 32 Dimethylacetophenone 85 Dimethylanilines, substituted 85 Dimethylbiphenyls 82 Diolefins 92 94 Dioxane 52 Diphenyilbutadiene 106 Diphenyl sulphone 143 Diphenylamine 42 Diphenylmethane dyes 89 Diphenylmethylene anthrones 108 Diphenylphosphate 143 Diphenylpolyacetylenes 36 Diphenylpolyenes 33 Dipole-dipole forces 140 Dipole-polarization forces 140 Dispersion curve 137 Dispersion, molecular 137 Dispersive interactions 140 Dissociation, homolytic 2 Dixanthogen 19 Dixanthylene 109 Diynes 27 Dodecapentaenic acid 142 Double bond, effect of twisting 88 Drude equation 137 Dyestuffs 134 137 Dyestuffs, fluorescence 102 Elaeostearic acid 74 Electron affinity 113 115 116 Electron affinity, high 114 Electron excitation 112 116 Electron excitation, energy 113 Electron-acceptation volume 118 Electron-resonance spectra 134 Electronegativity 20 Electronic interactions, decreased 82 Electronic interactions, increased 89 Electronic interactions, new 89 Electronic transitions, classification 11 Electrostatic model 125 Emission spectra 121 Emission spectra, low-temperature 108 Empirical regularities 47 Energy levels for polyatomic molecules 2 Energy states 114 Enones, 67 Enthalpy 118 120 entropy 118 Enyne alcohols 27 Enynes 27 Enzyme reactions 79 Epimerizations 138 Equilibria, monomer-dimer 143 Equilibrium constant 118 120 142 Equilibrium constant for complex formation 119 Ergosterol 26 73 78 Ergosterol-D 26 ethane 15 Ethers 93 100 Ethyl acetoacetate 70 Ethyl allene 37 Ethyl azide 37 Ethyl dithioacetate 19 Ethyl isothiocyanate 37 ethylene 10 11 93 Ethylene complexes with silver ion 121 Ethylene oxide 52 92 Ethylene oxide derivatives 90 Ethylene trithiocarbonate 20 23 Ethylene, substitution of 16 Ethylenes, alkyl-substituted 16 Ethylenes, substituted 108 Ethylidene-acetone 29 Exciton effect 102 Expansion of valence shells 42 Extinction 3 Extinction coefficient 3 113 119 Extinction coefficient, deviations 3 F-centres 131 Ferrocene 49 Field limit, strong 128 Field limit, weak 128 Flavones 135 Fluorescein 99 106 Fluorescence 98 Fluorescence of inorganic compounds 104 Fluorescence of metallo-organic compounds 104 Fluorescence of organic compounds 100 Fluorescence spectra, photoelectric measurement 100 Fluorescence, dependence on pH 105 Fluorescence, low-temperature 107 Fluorescence, polarized 107 Fluorescence, solvent effects 103 Fluorescent light, polarization 107 Fluorobenzenes 47 Formation constants of complexes, successive 79 Franck — Condon principle 2 108 140 Frequency shifts, infra-red 111 Frequency shifts, Raman 111 Furan 52 102 Glyoxal 32 Guanine 58 Haemin 135 Haemoglobin 135 Halides of metals 108 Hammett reactivity constants 44 Heat of formation 79 114 Heat of formation of complexes 113 Heteroaromatic compounds 22 Heterocyclic compounds 52 Heterocyclic compounds, six-membered aromatic 53 Heterocyclic compounds, unsaturated five-membered 52 Hexachlorobiphenyl 87 Hexamethylbenzene 84 Hexatriene 25 Hydration equilibria 79 Hydrocarbons, benzenoid 48 Hydrocarbons, catacondensed 95 Hydrocarbons, monoaromatic 48 Hydrocarbons, saturated 92 100 Hydrocarbons, unsaturated 92 Hydrogen bonding 115 141 142 149 Hydrogen bonding forces 140 Hydrogen bonding in proteins 148 Hydrogen bonding, intermolecular 143 Hydrogen bonding, intramolecular 143 Hydrogen bonding, solute-solvent 143 Hydronaphthaquinone 63 Hydroxindanones 65 Hydroxybenzoic acid 143 Hydroxynaphthalenes, ionic 108 Hydroxynaphthalenes, molecular 108 Hydroxypyrene sulphonic acids 105 Hyperchromic effect 12 Hyperconjugation 16 18 20 41 Hypochromic effect 12 Hypsochromic shifts 12 Imidazole 52 Imides, cyclic 96 Indigo dye 102 Indole 53 Indole ions 102 Inductive effects 17 20 40 44 Inorganic compounds, colour centres 131 Inorganic compounds, fluorescence 104 Instruments 4 Instruments, calibration 6 Insulin 148 149 Intensity enhancement 117 Intensity factor 115 Intercarbonyl angles 33 Iodine complexes 114 Iodine complexes with benzene 119 120 iodobenzene 101 Ion pairs 113 114 Ionic contribution 116 Ionization bands 92 Ionization energies 15 Ionization potential 113—116 Ionization, homolytic 2 Ionones 66 Ions, inorganic 22 Irone 29 Iso-absorptive point 79 Isobestic point 74 79 142 Isobutenylvinylcarbinol 68 Isoduril 33 Isoflavones 135 Isomerism, cis-trans 69 Isomerism, optical 70 Isomerization reactions 78 Isomers, cis-trans 106 Isomers, cis-trans, photochemical isomerization 106 Isomers, geometrical 69 Isomethylbixin 101 Isomycomycin 65 Isopropene 26 Isoquinoline ions 102 Isoquinolium ions 102 Isothujone 29 Jahn — Teller effect 130 Ketelaar equation 119 Ketones 75 Ketones, 28 Ketones, acetone 96 Ketones, alicyclic 18 19 Ketones, aliphatic 17 92 96 101 Ketones, asymmetric 138 Ketones, cyclopropyl 96 Ketones, steroid 138 L-Pimeric acid 26 Lanthanum chloride matrix 105 Levopimaric acid 66 Lewis acid, complexes with polyene 134 Ligand field stabilization energies 129 Ligand field theory 124 Ligands, in spectrochemical sequence 130 Linkages, isolated ethylenic 16 Linkages, metal-olefin 121 Linkages, nitrogen-nitrogen 22 Linkages, nitrogen-oxygen 20 Linkages, peptide 148 Linkages, substitution of ethylenic 16 Linoleic acid 74 Linolenic acid 74 Lycopene 135 Lysozyme 149 m-Cresosulphonaphthalein 143 Malachite green 89 103 Manganese halides 104 McRae expression 141 Menthadiene 26 Mercaptotetrazoles 72 Mercaptothiazoles 72 Mesityl oxide 13 27 29 Mesitylene 87 95 Meso-ionic compounds 58 Mesoporphyrin 104 meta-Polyphenyls 35 Metals, halides 108 methane 11 15 Methyl ethyl ketone 30 101 Methyl heliotrope O 103 Methyl i-propyl ketone 18 Methyl-iso-propenyl-ketone 29 Methylamine 11 Methylene blue 103 Methylvinyl-ketone 29 Michler’s hydrol blue 89 Microscopes, reflecting 8 Millimicron 1 Mixtures analysis 73 Mixtures, binary 79 Molecular weight determination 74 Molecular weight of aldehydes 75 Molecular weight of amines 75 Molecular weight of ketones 75 Molecular weight of saturated alcohols 75 Molecular weight of sugars 75 Molecules, aromatic 39 Molecules, conjugated 25 Molecules, inorganic 22
Реклама
 |
|
О проекте
|
|
О проекте



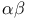 -unsaturated
-unsaturated