|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Hume-Rothery W. — Atomic Theory for Students of Metallurgy |
|
|
 |
| Предметный указатель |
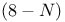 rule 100 145 272 rule 100 145 272
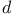 -states 79 -states 79
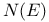 curves 94 158 186 198 230 234 243 252 260 269 270 curves 94 158 186 198 230 234 243 252 260 269 270
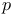 -bonds 136 -bonds 136
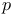 -states 76 -states 76
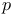 -states, in solid metals 210 -states, in solid metals 210
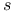 -polyhedron 218 -polyhedron 218
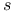 -states 72 -states 72
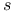 -states, in solid metais 209 -states, in solid metais 209
Absorption spectrum 14
action 19 27
Action, principle of least 26 32
Alkali metals 151 213 249
Alkali metals, alloys of 215
Alkali metals, compressibility 224 227
Alkali metals, lattice spacings 215
Alkaline earth metals 249
Alpha particles 5
Aluminium 251
Aluminium, 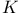 emission spectrum 92 emission spectrum 92
Aluminium, 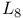 emission spectrum 92 emission spectrum 92
Aluminium, atomic diameter 254
Aluminium, Brillouin zones 252
Andrews, K. W. 238
Angstrom unit 7
Antimony, crystal structure 99 123
Aston, F. W. 4
Atomic cell 218
Atomic diameter 109 216 246 260 258
Atomic number 5 53
Atomic polyhedron 218
Atomic structure, 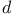 -states 79 -states 79
Atomic structure, 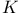 emission spectrum 92 emission spectrum 92
Atomic structure, 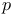 -states 76 -states 76
Atomic structure, 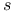 -states 72 -states 72
Atomic structure, electron cloud density 66
Atomic structure, electron groups 50
Atomic structure, electronic structure, tables 56
Atomic structure, meaning of quantum numbers 50 62
Atomic structure, probability pattern 50
Atomic structure, radial electron density 67
Atomic unit of length 7 72
Atomic weights, table 3
Avogadro 3
Avogadro’s number 3
Benzene, etnietuTe 144
Bernal, J. D. 116 233
Beryllium, Brillouin zone 24
Beta-rays 5
Binding energy 220
Bismuth 99 272
Bismuth, magnetic properties 282
Blackman 263
Bloch, F. 149 279
Body-centred cubic structure 101
Body-centred cubic structure, 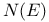 curves 234 252 curves 234 252
Body-centred cubic structure, Brillouin zone 214 231 234 239
Bohr magneton 10 63 177 268
Bohr, N. 7 14ff. 63
Boltzmann 9
Boltzmann’s constant 9
Born 106
Bradley, A. J. 114
Bragg reflection of electrons in crystals 190 193
Brass,  equilibria 115 229 equilibria 115 229
Brass,  112 114 231 112 114 231
Brass,  112 114 232 112 114 232
Brillouin zones 179 ff. 214 230
Brillouin, L. 179
Cadmium 102
Cadmium, 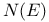 curves 243 curves 243
Cadmium, Brillouin 247
Cady, W. 243
Caesium 213
Caesium chloride structure 117
Calcium fluoride, structure 107
Channel — Evans, K. M. 109 114 233
Characteristic temperature 264
Close-packed hexagonal structure 101
Close-packed hexagonal structure, Brillouin zone 241
Co-valent bonds 98 126 136
Compressibility 223 227
Compton effect 18
Compton, A. H. 18
Conductivity see Defect conductivity Electrical Thermal
copper 226 ff.
Copper alloys, theory 229
Copper, correlation forces in 134
Copper, lattice spacing 229
Copper, solid solutions in 260
Copper-eluminium alloys, theory 235
Copper-gallium alloys 116 237
Copper-germanium alloys 238
Copper-gold alloys 228
Copper-mdium alloys 237
Copper-rinc alloys, equilibrium diagram 109
Copper-rinc alloys, theory 235
Copper-silver alloys 228
Copper-tin alloys 114 237
Correlation forces 124 223
Crystal Angstroms 7
Curie point 276
Cyclical model 167 181
Dalton, J. 1
Davisson, C. 22
Daw, H. 1
de Broglie, L. 28
Debye, (The) 106
Debye, P. 263
Defect conductivity 148
Degeneracy 51
Diamagnetism 274 281
Diamagnetism, of free electrons 178
Diamagnetism, of ions 177
Diamond, structure 100 139 196 233
Dipole moment 105
Dirac, P. A. M. 21 72 76 78 161
Directed valence 136
Dulong 264
Dulong and Petit’s law 264
Effective electronic mass 201 209
Eiectron-cloud pattern 68 74
Einstein , A. 263
Ekman, W. 114
Electrical conductivity 96 148 170 198
Electrical conductivity, free-electron theory 170
Electrical conductivity, of transition elements 204 261
Electrical conductivity, zone theory 193
Electrical resistance, of solid solutions 204
Electrical resistance, of superlattices 206
Electrical resistance, of transition elements 204
Electrochemical factor 107 117
Electron bands 87 260 269
Electron compounds 118 116 232
Electron compounds, theory 232
Electron diffraction 24
Electron volt 7
Electron(s) 4
Electron(s), charge 4
Electron(s), free, diamagnetism 178
Electron(s), free, theory 149 151
Electron(s), mass 4
Electron(s), specific heat 158 163 265
Electron(s), spin 51 64
Electron(s), thermal conductivity 173
Electron(s), wave-lengths 22
Electron(s), wave-like properties 22 24
Electron-concentration rule 114
Electronic charge 4
Electronic emission 173 ff.
| Electronic structure, tables 66
Emission band 87
Emission Spectrum 14 83
Exchange forces 125 131
Exchange repulsion 134
Exclusion principle see Pauli principle
Exohange internal 277
Face-centred cubic structure 101
Face-centred cubic structure, 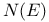 curve 234 252 curve 234 252
Face-centred cubic structure, Brillouin zone 185 228 231 234 239
Faraday 1 274
Faraday, (The) 4
Fermat 2
Fermat's principle 27 32
Fermi energy 167
Fermi surface 158 170 181 203
Fermi — Dirao statistics 151 159
Fermi, E. 151
ferromagnetism 274 277
Ferromagnetism, domain theory 280
Fowler, R. H. 152
Free-electron theory 149 151
Frohiioh. H. 2
Fuchs, K 227 239
Gamma-rays 6
Gas constant 10
Germanium 139 233
Germer, L. H. 22
Gold 226 ff.
Gold, lattice spacing 229
Gold-silver alloys 205 228
Goudsmit, S. 64
Greene, J. B. 269
Gregory, C. H. 114
Group velocity 68 142 181 188
Guggenheim, E. A.. J 52
gyromagnetic ratio 276
Hail effect 202
Hall 202
Hamilton 28
Heat of sublimation see Binding energy
Heisenberg's principle 18 43 75 153 284
Heisenberg, W. 18 21 43 284
Heusler alloys 278
Homopolar bonds see Co-valent bonds
Hume - Rothery rule see Electron-concentration rule
Hume — Rothery, W. 109 114 229 238 239 245 268
Huy gens 23
hybridization 88 267
Hydrogen atom, 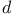 -states 79 -states 79
Hydrogen atom, 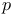 -states 76 -states 76
Hydrogen atom, 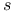 -states 72 -states 72
Hydrogen atom, Bohr theory 14 ff.
Hydrogen atom, early theory 11
Hydrogen atom, exact meaning of quantum numbers 62
hydrogen molecule 126 ff. 142
Indeterminancy principle see Heisenberg’s principle
Indium, crystal structure 102
Inert gases 97
insulators 96 101 148 192
Intermediate phases of alloys 111
Intermetailio compounds 112
Iodine, crystal structure 98 123
ionic bond 82 105
Ionic radii 249
Ionisation potential 57 220
Iron, electron bands in 269
Isotopee 5
Jones, H. 162 156 179 226 233 239 241 246 261 260 272
kX. unit 7
Land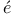 splitting factor 277 splitting factor 277
Lewis, G. N. 97 107 125
Light, quantum theory 18 23
Light, wave theory 23
Lithium, 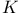 emission spectrum 92 emission spectrum 92
Lithium, theory of solid 213 ff.
Long-range order 110 117
Mabbott, G. W. 109 114 233
Magnesium alloys, lattice spacing relations 244
Magnesium, 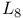 emission spectrum 92 emission spectrum 92
Magnesium, 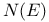 curve 243 curve 243
Magnesium, Brillouin zone 241
Magnesium-aluminium alloys 244
Magnesium-indium alloys 244
Magnetic susceptibilities, table 275
Magnetism 274 ff.
Manganese,  , Brillouin 232 , Brillouin 232
Manganese,  , structure 114 , structure 114
Manning, M. F. 269
Mass-energy relation 15
Matyas 253
Maupertuis 26
Mendeleev 1
Mercury, crystal structure 102
Metallic bonding 146
Molecular phase space 153
Momentum digram 153
Moseley 6
Mott, N. F. 162 156 179 226 243 251 260
Newton, Sir Isaac 23
Nickel 57
Orbital 126
Orbital hybrid 88 139 267
Paramagnetism 274 276 280
Paramagnetism, free-electron theory 177
Paramagnetism, of ions 177
Paschen~Beck effect 64
Pauli principle 52 125 153
Pauli, W. 52 126
Pauling, L. 140 147 249 260
Peierle, R. 179
Periodic field, electrons in 182 ff.
Periodic table 1
Petit 264
Phase space 152
Photoconductivity 196
Photoelectric emission 174
Photon 18 23
Physical constants 10
Planck 7 8
Planck’s constant 7 19
Polar bond see Ionic bond
Polar diagrams 69
Polarization force see van der Waals force
Potassium 213
Primary solid solutions 108
Quantum number 14 15 60
Quantum number, electron-spin quantum number 51 64
Quantum number, exact meaning of 62
Quantum number, molecular 125
Quantum theory 8 14
Radial electron density 67
Rare earth elements 57 274
Raynor, G. V. 229 239 245 272
Relativity, theory 15
Resonance bonding 82 142
Reynolds, P. W. 239
Rubidium 213
Rutherford 6
Rydberg 9 15
Rydberg constant 15
Rydberg unit 9
S 3
Schr dinger, E. 21 28 37 72 75 78 dinger, E. 21 28 37 72 75 78
Schrodinger equation 37 39
Screening constant 59
Seitz, F. 217
Selection rules 34
Selenium, atom 137
Selenium, crystal structure 99 138
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



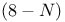 rule
rule  -states
-states 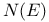 curves
curves 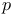 -bonds
-bonds 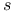 -polyhedron
-polyhedron 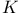 emission spectrum
emission spectrum 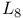 emission spectrum
emission spectrum  equilibria
equilibria 

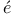 splitting factor
splitting factor  dinger, E.
dinger, E.