|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Fowler R.H. — Statistical thermodynamics: A version of statistical mechanics for students of physics and chemistry |

Обсудите книгу на
Нашли опечатку?
Выделите ее мышкой и нажмите Ctrl+Enter
|
Название: Statistical thermodynamics: A version of statistical mechanics for students of physics and chemistry
Автор: Fowler R.H.
Язык: 
Рубрика: Физика/
Статус предметного указателя: Готов указатель с номерами страниц
ed2k:
Год издания: 1956
Количество страниц: 701
Добавлена в каталог: 26.03.2008
Операции: Положить на полку |
Скопировать ссылку для форума | Скопировать ID
|
|
 |
| Предметный указатель |
Perfect gases 70 sqq.
Perfect gases, diatomic 82 sqq.
Perfect gases, diatomic, heat capacity, electronic for 102—106
Perfect gases, diatomic, heat capacity, rotational for 83
Perfect gases, diatomic, heat capacity, vibrational for 94—102
Perfect gases, diatomic, nuclear symmetry in 84—87
Perfect gases, diatomic, partition functions, rotational for 83
Perfect gases, diatomic, partition functions, vibrational for 97
Perfect gases, diatomic, thermodynamic functions for 88—90
Perfect gases, distribution laws for 73
Perfect gases, energy of 72
Perfect gases, equation of state of 71
Perfect gases, free energy of 71
Perfect gases, monatomic 80—82
Perfect gases, monatomic, heat capacity for 81
Perfect gases, monatomic, partition function for 80
Perfect gases, monatomic, partition function for, with nuclear spin 87
Perfect gases, monatomic, thermodynamic functions for 81
Perfect gases, number of impacts on a plane in 124
Perfect gases, partial pressure of 71
Perfect gases, polyatomic, heat capacity of 111—121
Perfect gases, polyatomic, internal rotations in 107—109
Perfect gases, polyatomic, partition functions for, final form 109—111
Perfect gases, polyatomic, symmetry numbers for 107
Perfect gases, polyatomic, thermodynamic functions for, final form 109—111
Perfect gases, polyatomic, vibrational freedoms for 107 sqq.
Perfect gases, pressure of 71
Perfect solutions 353—355
Perrin’s determination of Avogadro’s number 77
Phase equilibrium, condition for 64
photoelectric effect 481—489
Pipe field 640 671
Planck’s constant 3
Planck’s law of temperature radiation 130
Poisson — Boltzmann equation, refined solution of, in electrolytes 407—409
Polarization, molar optical, orientational, and total, defined 642—644
Potential energy of imperfect gases, partition function for 256—259
Quasi-chemical method for order 576—681
Quasi-chemical method, equivalent to Bethe’s method 595—598
Quasi-static process, defined 255
Radiation, partition function for temperature 129
Radiation, Planck’s law for temperature 130
Raman spectra of liquids 321
Raoult’s law for perfect solutions 354
Rayleigh calculation of surface energy 446
Reactions between crystals, transition points of 198
Reactions in gases, bimolecular see bimolecular reactions
Reactions in gases, classified 499—501
Reactions in gases, mechanism of, defined 500
Reactions in gases, order of, defined 499
Reactions in solutions, use of activated complex 535
Rectilinear diameter 318
Reduced equation of state 316
Refractive index of gases and liquids 641—643
Refractive index of imperfect gases 650
Regular assemblies, defined 245
Regular assemblies, grand partition function for 246—251
Regular assemblies, thermodynamic functions for 251—253
Regular solutions 351 355—366
Regular solutions Crude theory 356—358
Regular solutions refined theory 358—366
Regular solutions, defined 351
Regular solutions, partial vapour pressure of 358
Residual energy 41
Richardson’s emission formula for electrons 478
Rigid body, classical rotational partition function for 106
Rigid rotator 28
Rotational partition function for 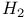 at low temperatures 91—94 at low temperatures 91—94
Rotational partition function, classical, for rigid body 106
Rotations, free, in crystals 151
Rotator, rigid 28
Rule of equal areas 315
Schroedinger’s equation 5 sqq.
Schroedinger’s equation for systems in external fields 610
Sedimentation 77
Semi-conductors 467
Simon and others heat capacity, anomalous, of 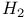 207 207
Simon and Simson rotations in crystals 151
Simon proof of third law 225
Simon, Nernst’s heat theorem 223
Solutions of electrolytes see electrolytes
Solutions of H in metals 554—563
Solutions of H in Pd 558—563
Solutions of molecules of widely different size 366—370
Solutions, activated complex in, used 535
Solutions, bimolecular reaction rates in 530—536
Solutions, collisions in 531—534
Solutions, encounters in 534
Solutions, entropy of 217—219
Solutions, ideal of impurities in crystals 552
Solutions, ideal, dilute see dilute solutions
Solutions, strictly regular see regular solutions
Sommerfeld electron theory of metals 452
Specific interaction, Bronsted’s principle of 417
Spectroscopic stability 619
Sphere field 640 671
Spreading pressure, thermodynamic theory of 422
Stark effect, crystalline 626
| Stark effect, linear and quadratic 611
State, stationary, of an assembly, defined 4—7
Statistical construction of Xx 234
Statistical mechanics relationship to thermodynamics in dissociating assembly 160—162
Statistical temperature scale 37—39
Steepest descents, method of 34—37
Stoner collective electron ferromagnetism 678—685
Stoner susceptibilities 608 sqq.
Structureless particles, partition function for, in a box 52—54
Superlattice lines, X-ray 564
Superlattice, defined 564
Surface energy of liquid-vapour interface 448—451
Surface tension near critical point 450
Surface tension, thermodynamic theory of 422
Surfaces 421 sqq.
susceptibility see electric е. diamagnetic magnetic
Symmetrical Eigen functions 16
Symmetry number for polyatomic molecules 107
Symmetry number, defined 87
Symmetry type for actual assemblies 17
System degenerate 50
System, defined 1
Temperature, absolute, defined 57
Temperature, absolute, measurement of, below 1° K. 665
Temperature, absolute, principles of measurement of 662—665
Temperature, absolute, scale of 39
Temperature, statistical scale of 37—39
Thermal expansion, anomalous for metallic alloys 606
Thermionic emission of electrons 476—481
Thermionic emission of electrons, experimental data 479—481
Thermionic emission of electrons, temperature dependence of 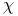 and and 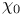 484—486 484—486
Thermodynamic functions for crystals 137—140
Thermodynamic functions for crystals, molecular 150—154
Thermodynamic functions for crystals, perfect mixed 184
Thermodynamic functions for dissociating assembly 160—162
Thermodynamic functions for electron gas 461—463
Thermodynamic functions for imperfect gases 256—259 265—267
Thermodynamic functions for liquids and compressed gases 342—345
Thermodynamic functions for liquids, crude models 328
Thermodynamic functions for perfect gases 81 88 109
Thermodynamic functions for regular assemblies 251—253
Thermodynamic functions with magnetic interactions 658—660
Thermodynamic functions, extrapolation of, to absolute zero 192—194
Thermodynamic functions, special 232—234
Thermodynamic functions, statistical, compared with experiment 194—197
Thermodynamic potentials, defined 59
Thermodynamics for classical statistics 67
Thermodynamics in magnetic field 673
Thermodynamics, derived from statistical mechanics 62—65
Thermodynamics, laws of 55—62
Thermodynamics, second law of, alternative form 60—62
Thermodynamics, third law of 219—229
Thermodynamics, third law of, historical sketch 223
Thermodynamics, third law of, Nernst’s heat theorem deduced from 226
Thermodynamics, third law of, statistical interpretation 227—229
Tolman principles of statistical mechanics 7
Tolman theorem on bimolecular reactions 503
Tolman theorem on unimolecular reactions 521
Transition points of reactions between crystals 198
Transmission coefficient for electrons across barriers 478
Trouton’s rule 319 332—336 350
Uniform integrals and equations of motion 11
Unimolecular reactions, absolute rates of 529
Unimolecular reactions, rate constant for 519—521
Unimolecular reactions, reverse processes of 522
Unimolecular reactions, revised theory of rate of 526—529
Unimolecular reactions, temperature coefficient of rate of 521
Unimolecular reactions, theory and experiment compared for 523—529
Ursell’s method for evaluating 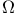 264 264
van der Waals equation of state 273
van der Waals theory of liquids 321
van Vleck susceptibilities 608 sqq.
Vapour pressure of crystals, general form of 181
Vapour pressure of crystals, isotopic mixed 186
Vapour pressure of hydrogen 205—210
Vapour pressure, constant 182
Vapour pressure, constant in practical units 197
Vapour pressure, constant of electron 476
Vapour pressure, constant, related to chemical constant 183
Vapour pressure, diatomic vapours 202—205
Vapour pressure, monatomic vapours 199—202
Vapour-crystal equilibrium 175—177
Vapour-liquid equilibrium 346—349
Vibrations of polyatomic molecules 107
Vibrations, distribution of energy of 495—497
Vibrations, heat capacity of see heat capacity
Virial coefficients 276
Virial coefficients, second compared with experiment 282—289
Virial coefficients, second for binary mixtures 296—300
Virial coefficients, second for polar molecules 294—296
Virial coefficients, second theoretical form of 279—282
Virial of Clausius 270
Volta potential 486—488
Weight 23—28
Weiss’s law for ferromagnetics above Curie point 677
Weiss’s law of paramagnetic susceptibility 627
Zeeman effect for atoms 616—618
Zeeman effect, linear and quadratic 611
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



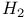 at low temperatures
at low temperatures  and
and