|
|
 |
| јвторизаци€ |
|
|
 |
| ѕоиск по указател€м |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Fowler R.H. Ч Statistical thermodynamics: A version of statistical mechanics for students of physics and chemistry |

ќбсудите книгу на
Ќашли опечатку?
¬ыделите ее мышкой и нажмите Ctrl+Enter
|
Ќазвание: Statistical thermodynamics: A version of statistical mechanics for students of physics and chemistry
јвтор: Fowler R.H.
язык: 
–убрика: ‘изика/
—татус предметного указател€: √отов указатель с номерами страниц
ed2k:
√од издани€: 1956
оличество страниц: 701
ƒобавлена в каталог: 26.03.2008
ќперации: ѕоложить на полку |
—копировать ссылку дл€ форума | —копировать ID
|
|
 |
| ѕредметный указатель |
External reactions of assembly 54
Eyring and Hirschfelder free volume of liquids 332
Eyring and others refined theory of reaction rates 514Ч517
Eyring entropy of liquids 327
Fermi Ч Dirac statistics 47 49 159
Fermi Ч Dirac statistics for electron gas 453
Ferromagnetism, collective electron, StonerТs treatment 678Ч685
Ferromagnetism, Curie temperature for 677
Ferromagnetism, HeisenbergТs explanation of 677
Ferromagnetism, ideal, laws for 674
Ferromagnetism, WeissТs theory of 675Ч677
Free energy in magnetic field 673
Free energy of adhesion between liquid and vapour 445Ч448
Free energy of electrolytes, DebyeТs approximation 392
Free energy of liquid, form of 322Ч324
Free energy of perfect gas 71
Free energy, configurational for non-equivalent sublattices 598Ч604
Free energy, configurational, power series for 574Ч576
Free energy, defined 58
Free volume of liquid 331Ч334
Fugacity, defined 266
Fundamental formula, defined 59
FuossТs treatment of ionic association 413Ч415
Gases, mixtures of, entropy of 162Ч164
Gases, mixtures of, free energy of 162Ч164
Gases, refractive index for 641Ч643
Giauque and others vapour pressure data 203
Giauque, comparison of calorimetric and spectroscopic entropy 196 210Ч215
Giauque, definition of ice point 69
Gibbs adsorption formula 423 444
Gibbs chemical potential 301
Gibbs condition for dissociative equilibrium 162
Gibbs definition of partial potentials 59
Gibbs elementary principles in statistical mechanics 55
Gibbs function 233
Gibbs grand canonical ensemble 231
Gibbs Ч Duhem relation 233 422
Gillespie and others solubility of H in Pd 561
Glasses, entropy of 217Ч219
Gorsky approximation for order 574
Grand canonical ensemble of Gibbs 231
Grand partition function 231 sqq.
Grand partition function for crystals 240Ч242
Grand partition function for crystals, general imperfect 552
Grand partition function for crystals, perfect mixed 242Ч244
Grand partition function for electron gas 667 681
Grand partition function for gases 236Ч239
Grand partition function for groups of sites 589Ч595
Grand Partition function for regular assemblies 244Ч253
Grand Partition function for surface phase, gaseous 239
Grand partition function, constructed and used 234
Gronwall and others solution of Poisson Ч Boltzmann equation 408
Guentelberg activity coefficients, ionic 407
Hamiltonian energy for rigid body 106
Hamiltonian, classical for systems in external field 608Ч610
HamiltonТs equations of motion 8
harmonic oscillator 25Ч28
Harmonic oscillator, partition function for 40Ч42
Heat capacity of crystals 141Ч149
Heat capacity of crystals DebyeТs approximation 141Ч144
Heat capacity of crystals, atomic, 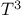 -law for 142 -law for 142
Heat capacity of crystals, molecular, 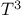 -law for 153 -law for 153
Heat capacity of hydrogen gas at high temperatures 98
Heat capacity of perfect gases 80 sqq.
Heat capacity of perfect gases, diatomic 90
Heat capacity of perfect gases, polyatomic 111Ч121
Heat capacity via partition functions 68
Heat capacity, anomalous configurational 583Ч586
Heat capacity, rotational, for 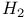 at low temperatures 91Ч94 at low temperatures 91Ч94
Heat capacity, vibrational slow attainment of equilibrium 100Ч102
Heat capacity, vibrational, for diatomic molecules at high temperatures 94Ч102
Heat of dilution, of electrolytes 403Ч405
Heat of dilutions, of solutions, dilute 374
Heat of evaporation of liquids 349
Heitler and London overlap energy 278
Helmholtz free energy 233
Henry heat capacity of simple gases 101
Herzfeld diameters of molecules 277
Heterogeneous reactions, equilibrium of 183
Hildebrand regular solutions, defined 245
Hildebrand rule 350
Hildebrand solutions of molecules of different lengths 370
Hinsheiwood unimolecular reactions 528
Hirschfelder and others entropy of melting 330
Hirschfelder and others equation of state at high pressures 289
Hirschfelder and others Joule Ч Thomson coefficients 288
Homogeneous reactions in Solutions, dilute 375
Homogeneous reactions, equilibrium of 156 sqq.
Hovorka and Rodebush osmotic coefficients 402
Hueckel electrolytes, theory of 394
Hund paramagnetic susceptibility 619
Hydration in electrolytes, effects of 379
Hydration in electrolytes, general discussion of 379Ч381
Ice point, GiauqueТs definition of 69
Ideal assemblies, defined 244
Ieotopic diatomic molecules, dissociation of 167Ч169
Impacts on a plane, number of, in perfect gas 124
Imperfect crystals 541 sqq.
Imperfect crystals of binary salts 545Ч549
Imperfect crystals of binary salts with excess of one component 549Ч552
Imperfect crystals, activation energy for transport in 549
Imperfect crystals, general, grand partition function for 552
Imperfect crystals, simple pure 542Ч545
Imperfect gases 255 sqq.
Imperfect gases at high pressures 289Ч291
Imperfect gases in external fields 300
Imperfect gases, chemical equilibrium in 271
Imperfect gases, dielectric constant of 650
Imperfect gases, distribution laws in 259Ч261
Imperfect gases, empirical equations of state for 273Ч276
Imperfect gases, general theory of 301 sqq.
Imperfect gases, partition function for, first approximation 262Ч265
Imperfect gases, partition function for, for potential energy 256Ч259
Imperfect gases, refractive index of 650
Imperfect gases, simple models 272
Imperfect gases, thermodynamic functions for 256Ч259 265Ч267
Impurities, solutions of, in crystals 552
Insulators and metals, compared 465Ч469
Intermolecular energy 276 sqq.
Intermolecular energy, analysis of 291Ч296
Internal rotations in polyatomic molecules 107Ч109
Ionic association, BjerrumТs theory of 409Ч412
Ionic association, FuossТs treatment of 413Ч415
Ionic atmosphere, mean thickness of 393
Ionic diameter, effect of, in DebyeТs approximation 397
Ionic interaction, specific 415Ч420
Ionic strength of electrolyte, defined 393
Isomeric equilibrium 157
Isotherms for 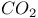 , observed 302 , observed 302
Isotherms LangmuirТs adsorption 427
Isotherms near critical temperature 314Ч318
Isotherms pressure-composition, for H in Pd 560Ч563
Isotherms, metastable 304 314
Isotopic mixed crystals, vapour pressure of 186
Isotopic mixtures, chemical constants of 174
Jahn and Teller theorem on crystalline fields 628
Joule Ч Thomson coefficient 266 288
Joule Ч Thomson coefficient for binary mixtures 299
Joule Ч Thomson coefficient, inversion temperature for 267
Kahn equation of state of gas 256 301
Kamerlingh Onnes and Keesom equations of state of gases 273
Keesom equation of state of gas 273
Keesom second virial coefficient for polar molecule 294
Kelvin temperature scale 39
Kinetic salt effects in bimolecular reaction rates 536Ч540
Kirkwood configurational free energy 574
Kirkwood critique of DebyeТs theory 406
Kramers crystalline fields, theorem on 627
Kramers electrolytes at high dilution 406
Lacher adsorption isotherm, refined theory 442
| Lacher solubility of H in Pd 558
Landau electron diamagnetism 669
Lange and Robinson heat of dilution of electrolytes 404
Langevin function 630 676
Langevin paramagnetic susceptibility, theory of 620
Langmuir adsorption isotherm 422 427
LarmorТs theorem 615
Lennard Ч Jonee and Devonshire free volume of liquids 332
Lennard Ч Jonee and Devonshire method of evaluating 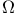 for liquids 323 for liquids 323
Lennard Ч Jones second virial coefficient 280
Lewis and Gibson third law of thermodynamics 224
Lewis fugacity, defined 266
Limiting principle 9 25 26Ч29
Lindemann unimolecular reactions 519
Liquids 319 sqq.
Liquids and vapour interface, surface energy of 448Ч451
Liquids and vapour, equilibrium of, refined model 346Ч349
Liquids relationship to gases and crystals 326
Liquids, associated, defined 320
Liquids, crude models of 324Ч326
Liquids, crude models of, thermodynamic functions for 328
Liquids, dielectric constant of 217Ч219 (see dielectric constant entropy of)
Liquids, free volume of 331Ч334
Liquids, harmonic oscillator model for 325
Liquids, heat of evaporation of 349
Liquids, normal, defined 319
Liquids, refined model of 336Ч350
Liquids, refined model of, corresponding states for 342Ч345
Liquids, refined model of, thermodynamic functions for 342Ч345
Liquids, refractive index for 641Ч643
Liquids, smoothed potential model for 325
Localized assemblies, complexions enumerated for 29
London dispersion energy 278
London second virial coefficient 296
Lorenz Ч Lorentz formula for refractive index 642
Macleod equation for surface tension 450
Magnetic field, free energy in 673
Magnetic interactions, effect of, on thermodynamic functions 658Ч660
magnetic susceptibility 608 sqq. (see also diamagnetic s. paramagnetic
Magnetic susceptibility of alkali metals 670
Magnetic susceptibility of metals 666Ч671
Magnetic susceptibility, atomic, defined 613
Magneton number 621
Matter, atomic constitution of 3
MaxwellТs law 73Ч77
Mayer equation of state of gas 276 301
Mechanism of gas reactions, bimolecular, defined 500
Mechanism of gas reactions, unimolecular, defined 500
Melting, elementary theory of 329Ч331
Melting, entropy of 329
Metallic alloys, order-disorder in 563 sqq.
Metals and insulators, compared 465Ч469
Metals, electron theory of 452 sqq.
Metals, magnetic susceptibility of 666Ч671
Metals, solutions of H in 554Ч563
Mixing, entropy and free energy of, in gases 163
Molecular energy, defined 72
Monolayer, ideal localized 426Ч428
Monolayer, ideal localized, spreading pressure for 443
Monolayer, mobile 423Ч426
Monolayer, regular localized 429Ч443
Monolayer, regular localized, crude approximation 430Ч433
Monolayer, regular localized, grand partition function for 438Ч441
Monolayer, regular localized, refined treatment of 437Ч443
Monolayer, regular localized, two-phase 433Ч435
NernstТs formula for equilibrium constant 173
NernstТs heat theorem 92 184 191 223
NernstТs heat theorem, conditions for validity of 226Ч229
NernstТs heat theorem, deduced from third law 226
NernstТs identification of vapour pressure and chemical constants 183
NernstТs principle of unattainability of absolute zero 224
Non-localized assemblies, complexions enumerated for 30
Normal modes of crystals 130Ч134
Normal modes of crystals, BoraТs analysis of 152
Normal modes of crystals, Debye terms 151
Normal modes of crystals, Einstein terms 151
Normal modes of elastic continuum 127
Nuclear spin 84Ч87
Nuclear spin in atomic partition functions 87
Nuclear symmetry in diatomic molecules 84Ч87
Onsager critique of DebyeТs theory of electrolytes 405
Order, long range, defined 567
Order-disorder comparison of approximations 581
Order-disorder comparison of theory and experiment 586Ч589
Order-disorder in metallic alloys 563 sqq.
Order-disorder quasi-chemical method 576Ч581
Order-disorder, Curie temperature for 573
Order-disorder, Curie temperature for, variation with composition 589
Order-disorder, variations of type of 605
Order-disorder, Zeroth approximation for 570Ч574
OrderЧdisorder, Zeroth approximation for, for 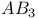 lattices 600Ч603 lattices 600Ч603
Orientational degeneracy frozen in 220Ч223
Ortho-molecules, defined 91
Ortho-para separations, effect of, on equilibrium 187
Oscillators, localized, assemblies of 30Ч34
Osmotic coefficients from DebyeТs approximation 399Ч401
Osmotic coefficients in electrolytes 381Ч383
Osmotic coefficients, compared with experiment 401Ч403
Overlap energy 277
Para-molecules, defined 91
Paramagnetic salts below 1∞ K. 661
Paramagnetic salts, entropy of 652
Paramagnetic saturation 629
Paramagnetic susceptibility in crystalline field 626Ч629
Paramagnetic susceptibility of atoms 618
Paramagnetic susceptibility of electron gas 667
Paramagnetic susceptibility of free molecules 631Ч633
Paramagnetic susceptibility of rare earth salts 621Ч624
Paramagnetic susceptibility of transition element salts 624
Paramagnetic susceptibility, CurieТs law for 619
Paramagnetic susceptibility, defined 610
Paramagnetic susceptibility, HundТs formula for 619
Paramagnetic susceptibility, LangevinТs formula for 620
Paramagnetic susceptibility, WeiseТs law for 627
Partial molecular volume, defined 72
Partial potential of crystal, final proof 178
Partial potential, defined 59
Partial pressure of perfect gas 71
Partition function for configurations of given order 568
Partition function for continuum 129
Partition function for crystals, DebyeТs approximation 133
Partition function for crystals, electronic factors for 134
Partition function for crystals, nuclear factors for 134
Partition function for crystals, perfect mixed 154
Partition function for electrolytes, dimensional analysis 384
Partition function for electrolytes, effect of electrostatic forces on 383
Partition function for electrolytes, MilnerТs formula 384
Partition function for electron spins in crystalline field 657
Partition function for electronic degrees of freedom 79 102Ч106
Partition function for harmonic oscillators 40Ч42
Partition function for imperfect gases first approximation 262Ч265
Partition function for imperfect gases, evaluated by association theory 267Ч270
Partition function for imperfect gases, structure of, general theory 301 sqq.
Partition function for perfect gases see perfect gases
Partition function for rigid rotators 43Ч45 106
Partition function for rigid rotators with electric dipoles 633 635
Partition function for rigid rotators with magnetic dipoles 620 629 652
Partition function for structureless particles in a box 52Ч54
Partition function for systems of several freedoms 39
Partition function for systems of several freedoms in external fields 612
Partition function for temperature radiation 129
Partition function, classical, for rigid body 106
Partition function, defined 33
Partition function, grand, further generalization of 253
Partition function, rotational, for 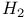 at low temperatures 91Ч94 at low temperatures 91Ч94
Pauli exclusion principle 17
Pauli theory of electron paramagnetism 667
Pauling entropy of ice 214
Pauling rotations in solids 151
Peierls order in non-equivalent sublattices 603
|
|
 |
| –еклама |
 |
|
|
 |
 |
|
 |
|
ќ проекте
|
|
ќ проекте



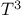 -law for
-law for 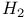 at low temperatures
at low temperatures 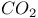 , observed
, observed 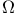 for liquids
for liquids 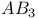 lattices
lattices