|
|
 |
| јвторизаци€ |
|
|
 |
| ѕоиск по указател€м |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Ferziger J.H., Kaper H.G. Ч Mathematical theory of transport processes in gases |
|
|
 |
| ѕредметный указатель |
Gakhov, F. D. 477 553
Gandhi, J. M. 296 553
Garcia-Colin, L. S. 6 387 390 395 396 398 553
Gas constant 16
Gas-surface interaction 77Ч79 469Ч472
Generalized Boltzmann equation, for dense gas 380
Geometrical collision variables see "Collision variables" "Deflection
Gibson, R. O. 372 556
Goldman, R. 51 421 553
Goldstein, H. 33 85 553
Gorter, C. J. 351 553
Grad, H. 6 87 102 114 122 132 151 154 159 161 162 553
Gradient vector 497
Gray, P. 290 552
Green, H. S. 36 550
Green, M. S. 6 23 387 390 395 396 398 403 553
Grew, K. E. 287 553
Gross, E. P. 467 550 553
Guernsey, R. 424 554
Guiraud, J. P. 79 97 154 552 554
Gyrofrequency 429 453 455
Gyromagnetic radius 429 430
gyromagnetic ratio 351
H-function 71 79
H-function, behavior at local Maxwellian state 86
H-function, connection with entropy 75
H-function, information-theoretical interpretation of 75
H-theorem, for Boltzmann's equation 72 83
H-theorem, for generalized Boltzmann equation, absence of 386 392
H-theorem, for Waldmann Ч Snider equation 334
H-theorem, for Wang Chang Ч Uhlenbeck equation 315
H-theorem, interpretation of 70Ч71 75
Haines, L. K. 421 554
Hall current 444
Hamiltonian function 33 335 354
Hamiltonian operator 33 377
Hard molecule 239
Harris, E. G. 424 557
Heat conduction see "Thermal conductivity"
Heat conduction, Eucken's theory of, for polyatomic gas 307Ч312
Heat conduction, phenomenological theory of 198
Heat flow vector, Chapman Ч Enskog approximation, for binary gas mixture 190
Heat flow vector, Chapman Ч Enskog approximation, for dense gas, Enskog's theory 365 368Ч369
Heat flow vector, Chapman Ч Enskog approximation, for dense gas, general theory 391 399
Heat flow vector, Chapman Ч Enskog approximation, for gas mixture 169 177Ч179
Heat flow vector, Chapman Ч Enskog approximation, for ionized gas 444Ч445
Heat flow vector, Chapman Ч Enskog approximation, for Lorentz gas 195
Heat flow vector, Chapman Ч Enskog approximation, for polyatomic gas, quantum mechanical 348 352
Heat flow vector, Chapman Ч Enskog approximation, for polyatomic gas, quasi-classical 319
Heat flow vector, Chapman Ч Enskog approximation, for simple gas 121 123 130Ч131 149Ч150 152Ч153
Heat flow vector, definition of, for dense gas 386
Heat flow vector, definition of, for gas mixture 18
Heat flow vector, definition of, for polyatomic gas, quantum mechanical 335
Heat flow vector, definition of, for polyatomic gas, quasi-classical 315
Heat flow vector, definition of, for simple gas 16
Heat, specific see "Specific heat"
Hecke, E. 116 554
Hess, S. 354 554
Hilbert class see "Normal solutions of Boltzmann's equation"
Hilbert paradox 115 172
Hilbert space 100
Hilbert's method, for solving Boltzmann's equation 109Ч115
Hilbert's method, for solving Boltzmann's equation, relation with Chapman Ч Enskog method 121Ч122
Hilbert's uniqueness theorem 109Ч115
Hilbert, D. 4 5 88 93 108 109 467 552 554
Hille, E. 102 106 554
Hirschfelder, J. O. 176 207 233 238 251 253 256Ч259 262 304 311 371 372 554
Holway, L. H. 488 554
Hydrodynamic velocity, definition of, for dense gas 381
Hydrodynamic velocity, definition of, for gas mixture 17
Hydrodynamic velocity, definition of, for polyatomic gas, quantum mechanical 335
Hydrodynamic velocity, definition of, for simple gas 12
Hydrodynamics see "Fluid dynamics"
Hydrostatic pressure 15 16 18 365 391
Hydrostatic pressure, influence of gradient on diffusion see "Diffusion driving force"
Ideal gas law see "Perfect gas law"
Ideal hydrodynamic equations see "Euler equations"
Ikenberry, E. 132 554
Impact parameter 28
Impact parameter, reduced 252
Index of repulsion 239
Inelastic collisions see "Collisions"
Initial layer 154 158 see
Initial value problem see "Existence theory"
Intermolecular force, nature of, between charged molecules 236Ч237
Intermolecular force, nature of, between monatomic molecules 232Ч234
Intermolecular force, nature of, between polyatomic molecules 234Ч236
Intermolecular potential, and temperature dependence of transport properties 255
Internal energy, definition of, for dense gas 381
Internal energy, definition of, for gas mixture 19
Internal energy, definition of, for polyatomic gas, quantum mechanical 335
Internal energy, definition of, for polyatomic gas, quasi-classical 315
Internal energy, definition of, for simple gas 12
Internal energy, equation of see "Conservation equations"
Invariants, summational or collisional see "Collisional invariants"
Inverse collisions see "Collisions"
Inverse thermo-electric effect see "Electro-thermal effect"
Inversion temperature 226
Ionized gas, non-equilibrium 456
Irreversibility 50 58 69Ч70
Isotopes, self-diffusion in a mixture of 190
Isotropic tensor 344 495 see
Jackson, E. A. 467 553
James, E. M. 159 552
Jancel, R. 132 427 554
Jaynes, E. T. 75 554
Jeans, J. H. 312 554
Jeffreys, H. 491 554
Jensen's inequality 82
Jones, R. C. 226 553
Kagan, Yu. 334 554
Kahan, Th. 132 427 554
Kahn, B. 23 554
Katz, A. 75 554
Kawasaki, K. 421 554
Keller, J. M. 278 279 285 554
Kestin, J. 268 269 555
Kihara approximation 139Ч143 271Ч274 see "Thermal "Thermal "Viscosity"
Kihara model potential 242
Kihara, T. 242 555
Kim, S. K. 375 555
Kinetic energy, molecular 12
Kinetic theory, historical summary 3Ч8
Kinetic theory, outline of 1Ч3
Kinetic theory, relation with fluid dynamics 153Ч162
Kirkwood, J. G. 36 555
Knudsen number 464
Kogan, M. N. 465 555
Kohler, M. 134 555
Kramer's problem see "Slip flow problem"
Kronecker symbol see "Unit tensor"
Krook, M. 467 550
Ku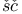 er, I. 161 467 555 er, I. 161 467 555
Kumar, K. 132 555
Ladyzhenskaia, O. A. 150 555
Lampis, H. 472 551
Landau, L. D. 231 260 261 263 555
Landoldt-B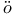 rnstein 302 555 rnstein 302 555
Langevin, P. 4
Laranjeira, M. F. 287 555
Lenard, A. 424 555
Lennard Ч Jones model potential 240 252 275Ч279 288
Lennard Ч Jones model potential, application to polyatomic gases 303
Lennard Ч Jones model potential, parameters for noble gases 276 284Ч285
Lennard Ч Jones model potential, transport integral ratios, numerical tables 528Ч532
Lennard Ч Jones model potential, transport integrals, numerical tables 522Ч525
Lennard-Jones, J. E. 240 555
Liepmann, H. W. 486 555
Lifshitz, E. M. 231 260 261 263 555
Linearized Boltzmann equation 87 465
| Linearized Boltzmann equation, existence and uniqueness theory for 97Ч106
Linearized collision operator 87Ч88 161 409
Linearized collision operator, BGK-model 467 484
Linearized collision operator, model construction 467Ч469
Linearized collision operator, properties of 88Ч96 465Ч466
Linearized ternary collision operator 410
Liouville equation 35 39 56
Liouville's law for elastic collisions 27
Loaded sphere 312
London, F. 233 555
Longmire, C. L. 430 555
Lorentz force 429
Lorentz gas 191
Lorentz gas, convergence of Chapman Ч Cowling and Kihara approximations 271Ч274
Lorentz gas, perturbation methods based on 196
Lorentz gas, transport properties 191Ч196
Lorentz, H. A. 4 191 555
Loyalka, S. K. 468 482 555
Lunn, A. C. 5 555
Macroscopic observables, vector of 113 116
Magnetic field, effect of, on transport properties, for ionized gas 444 445
Magnetic field, effect of, on transport properties, for polyatomic gas (Senftleben Ч Beenakker effect) 351Ч355
Magnetodynamics or magnetohydrodynamics or MHD 425
Magnetogasdynamic (or magnetohydrodynamic or MHD-) equations 433
Marshall, W. 430 440 449 555
Mason, E. A. 175 196 199 221 244 259 262 271 273 286 287 294Ч296 298 303 306 325Ч328 511 519 523 526 528 555Ч559
Mass density, definition of, for dense gas 381
Mass density, definition of, for gas mixture 17
Mass density, definition of, for polyatomic gas, quantum mechanical 335
Mass density, definition of, for simple gas 11
Mass, molecular 10
Mass, molecular, reduced 205
Massey, H. S. W. 231 556
Material derivative see "Substantial derivative"
Maximum principle 134 181
Maxwell molecules, 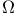 -integrals 251 -integrals 251
Maxwell molecules, definition of 239
Maxwell molecules, eigenfunctions of collision operator for 239 252 466 467
Maxwell, J. C. 3 4 64 74 556
Maxwellian velocity distribution function see "Equilibrium solution"
Maxwellian velocity distribution function, definition of 74
Maxwellian velocity distribution function, linearization with respect to 87 98
Maxwellian velocity distribution function, properties of 76Ч77
Mazur, P. 176 340 348 351 450 552
McCourt, F. R. W. 7 334 337 340 342 552 556
McCoy, B. J. 370 556
McCune, J. E. 116 556
McGee, I. J. 243 550
Mean collision time 51
Mean free path 19 20 154 158 197
Mean free time 19 108 154 158
Michels, A, 372 556
Mixing rules 244Ч245
Mixing rules, verification of, for Lennard Ч Jones (6Ч12) potential parameters 282Ч284
Model potentials 237 244
Modeling, of collision operator 467Ч469
Modified Buckingham (6-exp) model potential 241 252 275
Modified Buckingham (6-exp) model potential, transport integral ratios, numerical tables 519Ч521
Modified Buckingham (6-exp) model potential, transport integrals, numerical tables 511Ч518
Molecular chaos 58 379 see Stosszahlansatz"
Molecular fraction 219
Molecular fraction, dependence of transport properties on 280 290
Molecular interaction operator 35 377
Moment of inertia 329
Momentum, molecular 11
Momentum, molecular, equation of see "Conservation equations" "Angular
Monchick, L. 175 196 199 244 286 303 305 306 325Ч328 523 526 528 556
Monte Carlo method, for solving Boltzmann's equation 488Ч490
Montgomery, D. 424 556
Morgenstern, D. 5 153 556
Morse model potential 242 279 283
Morse model potential, transport integrals and transport integral ratios, numerical tables 534Ч535
Morse, T. F. 116 556
Mott, N. F. 231 556
Muckenfuss, C 229 312 552 556
Mundy, J. N. 287 553
Munn Ч Smith model potential 277 287Ч288
Munn, R. J. 259 262 277 286Ч288 534 556 557
Murphy, T. J. 402
Muskhelishvili, N. I. 478 556
Narasimha, R. 486 555
Navier Ч Stokes equations 131
Navier, C. L. M. H. 150 556
Neutron transport theory 468
Newton's law, of viscosity 68
Noble gas mixtures, transport properties of 279Ч298
Noble gases, transport properties of 274Ч279
Normal pressure 15
Normal solutions of Boltzmann's equation, class of 114
Number density, definition of, for dense gas 381
Number density, definition of, for gas mixture 17
Number density, definition of, for polyatomic gas, quantum mechanical 333
Number density, definition of, for simple gas 11
O'Neal, C 274 557
Ohm's law 443
Ohmic heating 433
Onsager relations 176 349
Onsager, L. 226 553
Oppenheim, I. 421 554
Orbiting collisions see "Collisions"
Order-of-magnitude estimates, of successive terms in Chapman Ч Enskog approximation 151Ч153
Pair correlation function 389
Pair distribution function 381
Partial bracket integrals see "Bracket integrals"
Partition function, for internal states of polyatomic molecules 316 317 336
Pauling, L. 233 557
Peculiar velocity, definition of 12 18
Pekeris, C. L. 93 557
Perfect (or ideal) gas law 77 112 123
Phase mixing operator 38 377
Phase shift 261
Phase space (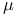 -space) 33 -space) 33
Phenomenological theory of transport properties 196Ч200
Phillips, R. S. 102 106 554
Pidduck, F. B. 312 557
Planck's constant 259 260 331
Plasma frequency 425
Plasma physics 423
Point center of repulsion model potential 239 272Ч273
Point center of repulsion model potential, 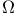 -integrals for 250Ч252 -integrals for 250Ч252
Poisson bracket 35
Polarizability, numerical table 302
Polarization 232
Polyatomic gas, classical theory 328Ч330
Polyatomic gas, Eucken's theory 307Ч312
Polyatomic gas, quantum mechanical theory 330Ч350
Polyatomic gas, quasi-classical theory 313Ч328
Position vector 10
Potential energy, effective 253
Povzner, A. Ja. 153 557
Pressure see "Hydrostatic pressure"
Pressure tensor, Chapman Ч Enskog approximation, for dense gas, Enskog's theory 365 368Ч369
Pressure tensor, Chapman Ч Enskog approximation, for dense gas, general theory 390 400
Pressure tensor, Chapman Ч Enskog approximation, for gas mixture 169
Pressure tensor, Chapman Ч Enskog approximation, for ionized gas 449Ч450
Pressure tensor, Chapman Ч Enskog approximation, for polyatomic gas, quantum mechanical 345 352
Pressure tensor, Chapman Ч Enskog approximation, for polyatomic gas, quasi-classical 319
Pressure tensor, Chapman Ч Enskog approximation, for simple gas 121 123 129Ч130 147Ч149 152Ч153
Pressure tensor, definition of, for dense gas 383
Pressure tensor, definition of, for gas mixture 18
Pressure tensor, definition of, for polyatomic gas, quantum mechanical 335
Pressure tensor, definition of, for polyatomic gas, quasi-classical 315
Pressure tensor, definition of, for simple gas 15
Pseudovector 497
Quantum theory of scattering 260Ч263
Range, of intermolecular force 50 377
Rankine Ч Hugoniot relations 485
Rarefied gas 152 464Ч490
Rate-of-shear tensor 129 345
Rate-of-strain tensor 129
|
|
 |
| –еклама |
 |
|
|
 |
|
ќ проекте
|
|
ќ проекте



 er, I.
er, I.  rnstein
rnstein  -integrals
-integrals 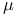 -space)
-space)