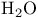|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Helgaker T., Jorgensen P., Olsen J. — Molecular Electronic-Structure Theory. Part 2 |
|
|
 |
| Предметный указатель |
Statistical analysis, reaction enthalpies SI 867
Statistical measures of errors 821
Statistical measures of errors, maximum absolute error 821
Statistical measures of errors, mean absolute error 821
Statistical measures of errors, mean error 821
Statistical measures of errors, normal distribution 539 821
Statistical measures of errors, standard deviation 539 821
Step-up and step-down operators 38
Stirling approximation for factorials 245 525
STO basis sets 227
STO basis sets, basis-set for carbon atom 229
STO basis sets, double-zeta (DZ) basis sets 228
STO basis sets, extended basis sets 228
STO basis sets, helium atom 262
STO basis sets, integrals for 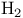 (table) 149 (table) 149
STO basis sets, minimal basis for 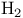 146 146
STO basis sets, minimal basis sets 228
STO basis sets, relation to nuclear cusp condition 261
STO basis sets, single-zeta basis sets 228
STO basis sets, Slater’s rules for exponents 228
STO, Slater-type orbital 226
STO-kG basis sets 288
STO-kG basis sets, scaling of exponents 289
STO-kG basis sets, STO-3G exponents 290
Symmetry constraints, linear variation method 135
Symmetry constraints, nonlinear variation method 136
Symmetry constraints, orbital rotations 89
Symmetry constraints, relation to instabilities in Hartree — Fock theory 497
Symmetry dilemma in Hartree — Fock theory 137 504
Time reversal 97 567
Total-spin operator 39 50 172 174 704 707
Translation matrix, complex translation matrix 411
Translation matrix, real translation matrix 413
Triplet instabilities see also “Singlet instabilities”
Triplet instabilities, general discussion 498
Triplet instabilities, Hartree — Fock dissociation of 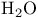 175 500 175 500
Triplet instabilities, Hartree — Fock dissociation of 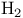 172 173 500 512 172 173 500 512
Triplet instabilities, removal of 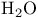 instability by MCSCF theory 640 instability by MCSCF theory 640
Triplet operators 42 (see also “Spin tensor operators”)
Trust-region method 479 610
Trust-region method, level-shift parameter 479 612
Trust-region method, level-shifted Newton step 479 612
Trust-region method, Levenberg — Marquardt trajectories 612
Trust-region method, step control 614
Trust-region method, trust radius 479 611
Trust-region method, trust region 479 611
Trust-region method, update of trust radius 615
Two-electron integrals see also “Molecular integrals”
Two-electron integrals in orbital basis 37 38
Two-electron integrals in spin-orbital basis 13 14
Two-electron integrals, Coulomb integrals 361
Two-electron integrals, molecular integral evaluation 336
Two-electron integrals, permutational symmetry in orbital basis 37
Two-electron integrals, permutational symmetry in spin-orbital basis 13
Two-electron integrals, separation in classical and nonclassical contributions 401
Two-electron integrals, upper bounds 404
Two-electron operators (in second quantization) 11
Two-electron operators (in second quantization) for nonorthogonal spin orbitals 29
Two-electron operators (in second quantization) for orthonormal orbitals 37
| Two-electron operators (in second quantization) for orthonormal spin orbitals 11
Two-level multipole method 420
Two-point extrapolation 322 842
Two-state model of perturbation theory 770 772
TZ basis sets 300
TZ, triple-zeta [basis set] 300
UHF, unrestricted Hartree — Fock [model] 170 435
Unitary matrix see also “Exponential matrix function”
Unitary matrix, definition 80
Unitary matrix, exponential form 81
Unitary transformation see also “Exponential unitary operator”
Unitary transformation of configuration states 630
Unitary transformation of creation and annihilation operators 86 88
Unitary transformation of spin orbitals 86
Unitary transformation of states in Fock space 89
Unlinked coupled-cluster equations 658
Unrestricted Hartree — Fock (UHF) theory 170 435 497
Up rank 660 663
Vacuum state 2 8
Vacuum state, annihilation operator applied to 5 28
Vacuum state, vacuum expectation values 19
Valence orbitals 301
van der Waals systems, 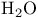 dimer 331 dimer 331
van der Waals systems, neon dimer 328
Variation method 112
Variation method for linear expansions 113
Variation method for nonlinear expansions 117
Variation method, Hylleraas variation method 734
Variation principle for exact states 108 11
Variation principle, upper bounds 115
Variational Lagrangian, CCPT Lagrangian 755 761
Variational Lagrangian, CI Lagrangian 616
Variational Lagrangian, coupled-cluster Lagrangian 674
Variational Lagrangian, general formulation 125
Variational Lagrangian, Hartree — Fock Lagrangian 458
Variational Lagrangian, MCSCF second-order Lagrangian 617
Variational Lagrangian, OCC Lagrangian 700
Variational Lagrangian, reduction to Hylleraas functional 734
Variational Lagrangian, RSPT Lagrangian 730
Variational Lagrangian, spin-restricted coupled-cluster Lagrangian 708
Vector-coupling coefficients 56
Vibrational energies 847
Vibrational energies, anharmonic constants 849
Vibrational energies, anharmonic contribution 849
Vibrational energies, force constants 11
Vibrational energies, harmonic approximation 847
Vibrational energies, harmonic contribution 849
Vibrational energies, harmonic frequencies 847
Vibrational energies, vibrational reference data (table) 850
Vibrational energies, zero-point vibrational energy (ZPVE) 847
Vibrational energies, ZPVE corrections to atomization energies (table) 855
Vibrational energies, ZPVE corrections to reaction enthalpies (table) 866
Virial theorem 121 428
Virtual orbitals 178 301 304 434 451 455 629 818
Wave operators 127
Weight function 358 359
Wigner’s 2n+1 rule 728 733 734
Wigner’s 2n+1 rule, 2n+2 rule 733 734
ZPVE, zero-point vibrational energy 847
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



 (table)
(table)