|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Seitz F. (ed.), Turnbull D. (ed.) — Solid State Physics. Advances in Research and Applications. Volume 16 |

Обсудите книгу на
Нашли опечатку?
Выделите ее мышкой и нажмите Ctrl+Enter
|
Название: Solid State Physics. Advances in Research and Applications. Volume 16
Авторы: Seitz F. (ed.), Turnbull D. (ed.)
Язык: 
Рубрика: Физика/
Статус предметного указателя: Готов указатель с номерами страниц
ed2k:
Год издания: 1965
Количество страниц: 451
Добавлена в каталог: 03.08.2014
Операции: Положить на полку |
Скопировать ссылку для форума | Скопировать ID
|
|
 |
| Предметный указатель |
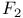 Center, energy levels, theory 195ff. Center, energy levels, theory 195ff.
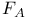 Center 131 Center 131
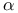 Center 169 181 Center 169 181
A Center 131
Abarenkov, L.V. 75
Abragam, A. 272
Ackerman, M. 344(3) 345(3) 346
Ackermann, R.J. 337(11) 338(11) 339 344(14) 345(14) 346
Addison, C.C. 326(33) 328
Additivity Rule, ionic separations 76
Adenstedt, H.K. 282(35) 283 314(19) 315(19) 316
Adkins, E.F. 326(29) 327(29) 328
Adrian, F.J. 91
Agron, P.A. 68
Ahlers, G. 341(49) 354
Ahrens, L.H. 79
Akishin, P.A. 344(6) 346
Al'tshuler, L.V. 412(7 11) 413(11) 414(7 11) 416
Al'tshuler, S.A. 272
Aldred, A.T. 345(43 44) 347
Alers, G.A. 380(1 7 9) 381(1 9)
Alexopoulas, K. 384(24) 385
Alkali chalcogenides, cohesive energy 63
Alkali halides, Born repulsive parameters 48
Alkali halides, Born — Mayer Repulsion 52
Alkali halides, cohesive energy, p. 42—59
Alkali halides, cohesive energy, p., Cohesive Energy, Table 54—56
Alkali halides, crystal data 44(T)
Alkali halides, dipole-dipole interaction 32
Alkali halides, F bands 126(T)
Alkali halides, face energy (001) 100
Alkali halides, Huggins — Mayer Repulsion 51
Alkali halides, ionic radii 86
Alkali halides, ionic separations 76
Alkali halides, M bands 206
Alkali halides, M bands, M Bands, Table 126
Alkali halides, N bands 126(T)
Alkali halides, optical dipoles 137
Alkali halides, R bands 126(T)
Alkali Ions, Screening Parameters 81
Alkali metals, bulk modulus 311
Alkali metals, bulk modulus, cohesive energy 311
Alkaline Earth chalcogenides, Cohesive Energy 63
Alkaline earths, bulk modulus 311
Alkaline earths, bulk modulus, cohesive energy 311
Altman, H.W. 315(33) 316
Anderson, C.T. 370(98) 374
Anderson, E. 326(27) 328
Angus, W.R. 361
Anthrop, D.F. 344(11) 346
Arajs, S. 352(86) 355 371(102) 372 375 384(4 8) 385
Arbogast, C.L. 380(2) 381
Argent, B.B. 315(29) 316 337(12) 338(12) 339
Arrott, A. 351(33) 353(33) 354 359
Asai, K. 157 160(61)
Asdente, M. 180 182(93)
Atomic Volume, elements, crystal 320ff.
Avakian, P. 130 132 208(20)
Aven, M.H. 351(26) 354 370(24) 372
Awane, K. 185
Bacon, G.E. 384(13) 385 387
Bailey, T.L. 55
Bakanova, A.A. 412(7 11) 413(11) 414(7 11) 416
Baker, J.A. 150
Baker, J.M. 255 259 263 269 271 272
Baldini, G. 174
Balk, P. 94 101 102 103
Bansigir, K.G. 218 219
Barron, T.H.K. 7 43(16) 352(82) 355 371(75) 374 380(3) 381
Barson, F. 315(26) 316
Barth 202 203 204
Barton, R.J. 338(41) 340 345(38) 347
Bates, D.R. 197
Batterman, B.W. 384(18) 385
Bauer, A.A. 327(40) 329
Baughan, E.C. 47 60
Beach, J.Y. 31
Beaudry, B.J. 326(16) 328
Beck, P.A. 35(27 36) 354 359 370(28 35) 373
Beecroft, R.I. 300(6) 303 304 306 307(36) 395
Bender, O. 281(1) 283 288(1) 290 292(1) 294
Benson, G.C. 15 21 23 68 94 98 99 100 101 102 103 106 119
Benson, G.W. 94 102
Berg, J.R. 332(10) 333(10) 334 363(7) 364(7) 365
Berg, W.T. 7 43(16) 380(3) 381
Berman, A. 352(84) 355
Bernstein, B.T. 282(37) 283 289(27) 290 293(25) 294
Bertaut, F. 15 21
Bethe, H.A. 272
Betterton, J.O., Jr. 351(25) 352(25) 353 370(25) 371(25) 373
Bhimasenachar, J. 281(30) 283 288(22) 290
Bilinsky, S. 388
Birch, F. 302(27) 304
Birchenall, C.E. 337(22) 339 344(17) 345(17) 346
Birman, J. 112 113
Blackburn, P.E. 337(13 15) 339
Blackman, M. 43 67 368 370(96) 371(96) 374 378 379 380(10 18) 381 382 383 384(9) 385 388
Blanpain, R. 352(97) 355 371(85) 374
Bleaney, B. 244 248 250 251 253 255 263 265 269
Bleick, W.E. 38
Bloomfield, P. 272
Blum, H. 130 150 177(49)
Blumberg, W.E. 5
Boiling point, elements 336ff.
Boiling point, elements, rare-earth metals 341
Bolef, D.I. 288(12) 290 292(11) 294 314(15) 316 319
Bonch-Bruevich, A.M. 200
Bonilla, C.F. 337(6) 339 344(18) 346
Boorse, H.A. 351(29 63) 352(29 84) 354 355 360 370(26 56 57) 371(26) 373
Booth, G.L. 370(32) 373
Born model, cohesion 1—120
Born model, crystal radii 84—88
Born repulsions 3
Born repulsive energy, ionic crystals, pp. 35—42
Born repulsive parameters, Alkali Halide Crystals 48
Born — Haber cycle 6 53
Born — Mayer repulsion 40 85
Born — Mayer Repulsion, alkali halide crystals 52
Born — Stern formula, face energy 100
Born, M. 2 3 6 8 30 34(18) 35 39 40 41 42 45 50 55 61 70(18) 71 72 92 99 103 104 106 107 113 118
Boswell, F.W.C. 95
Bowman, M.G. 344(8) 346
Boyd, G.E. 326(28) 328 362
Bragg numbers, elements 398ff.
Bragg, L. 40 76(64) 398
Braner, A.A. 130 189 190 194(110)
Brasted, R.C. 326(13) 327 328
Bravais lattice, cubic structure 12
Bredig, M.A. 68
Brewer, L. 344(2) 345(2) 346 347 369(57) 377(57)
Brickwedde, F.G. 325
Bridgman, P.W. 68 281(32) 283 288(24) 290 292(21) 294 298 299 300(1 2 3 4 5 7 8 10 11 12 14 15 16 17 18) 301(1 2 4 5 8 11 12 14 20 22 24) 302(1 2 4 5 8 12 20 22 26 28) 304 305 306 307
Brill, R. 60 384(16) 385
Brockhouse, B.N. 6 102(12) 384(19) 385
Broeklehurst, R.E. 315(34) 317
Bron, W.E. 130 146 150(40) 174 177 177 178 180(89) 182 188 189(40) 219(40)
Brown, F.C. 201 222
Brown, M. 360 370(56) 373
Bryant, C.A. 351(59 68 79) 352(79) 354 355 370(51 60 71) 371(71) 373 374
Buckingham, R.A. 31 33
Buckley, R.A. 326(27) 328
Buddery, J.H. 281(2) 283 288(3) 290 292(3) 294 314(2) 316 326(5) 328 337(3) 339
Budworth, D.W. 351(70) 352(70) 355 360 370(62) 371(62) 373
bulk modulus 280
Bulk modulus, cohesive energy correlation, elements 311
Bulk modulus, elements 298ff.
Bundy, F.P. 326(7) 328 332(4) 334 335
Bupp, L.P. 188
Burk, D.L. 351(7) 353 370(7) 372
Burstein, E. 131
| Butuzov, V.P. 306
Cable, J.W. 358
Cahill, J.A. 326(25) 328 337(21 32 33) 338(21 44) 339 340
Calcium fluoride, cohesive energy 67
Calcium fluoride, structure 11
Caldwell, W.C. 345(29) 346
Callaway, J. 348
Carlson, C.E. 281(21) 282(21) 283 288(17) 289(17) 290 292(17) 293(17) 294 301(25) 304
Carlson, O.N. 282(40) 284 289(29) 290 293(26) 294
Carnelley, T. 397
Carpenter, G.B. 385
Carter, W.J. 412(9) 413(9) 414(9) 416
Caspari, M.E. 169 186 187
Cauchy relation, calcium fluoride 67
Cauchy relation, ionic crystals 6
Central forces, ionic crystals 3
Cesium chloride, Madelung constant 25
Cesium chloride, structure 10
Cesium chloride, structure, face energy (011) 103
Cesium Salts, color centers 129(T)
Cesium Salts, M bands 207
Chalcogenides, cohesive energy 63
Chandrasekhar, B.S. 351(37) 352(83) 354 355 380(8 16 17) 381 382
Channel-Evans, K.M. 420
Cheng, C.H. 351(27 36) 354 359 370(28 35) 373
Child, H.R. 358
Chiotti, P. 282(40) 284 289(29) 290 293(26) 294
Chipman, D.R. 384(18) 385
Chiswik, H.H. 282(41) 284
Chopra, K.. 384(16) 385
Chou, C. 351(64) 352(95) 354 355 371(83) 374
Christensen, S.H. 150
Christian, J.W. 384(12) 385
Chupka, W.A. 344(4) 346
Clark, C.D. 184
Clark, C.W. 351(41) 354 360 370(38) 373
Clement, J.R. 351(78) 355 370(70) 374
Clougherty, E.V. 351(5) 353 359 370(4) 372
Clusms, K. 351(32 42 67 69) 352(67 69) 353 354 355 370(30 59 61) 371(59 61) 373
Cochran, W. 6 102(12)
Coffinberry, A.S. 282(43) 284 289(31) 290 293(28) 294 315(38) 317 327(44) 329
Cohen, E.R. 321
Cohesion, ionic crystals, pp. 1—120
Cohesive energy, alkali chalcogenides 63
Cohesive energy, alkali halide 42—59
Cohesive energy, alkali halide, Table 54—56
Cohesive energy, alkaline, earth chalcogenides 63
Cohesive energy, bulk modulus correlation, elements 311
Cohesive energy, calcium fluoride 67
Cohesive energy, chalcogenides 63
Cohesive energy, copper halides 61
Cohesive energy, elements 348ff.
Cohesive energy, elements, table 344
Cohesive energy, ionic crystals 6—74
Cohesive energy, rare-earth metals 341
Cohesive energy, silver halides 61
Cohesive energy, thallium halides 61
Color centers, alkali halides 126(T)
Colvin, R.V. 352(86) 355 371(102) 372 375 384(4 8) 385
Colwell, J.F. 73
Compressibility constants, elements 395(T)
Compressibility, adiabatic-isothermal relation 305
Compressibility, elements 298ff.
Compressibility, elements, zero pressure, Table 308
Compressibility, Grueneisen constant relation 305 394 411
Compton, W.D. 122 136 143 144 145 146(33 37) 147(33) 154(59) 155 156 157(59) 159 160 161 169 172 175(59 66) 176 184 200(7) 212(37) 220
Comrie, L.J. 110 118
Cooper, H.S. 281(5) 283
Copper halides, cohesive energy 61
Corak, W.S. 351(28 44) 352(44 81) 354 355 370(27 39) 371(39 74) 373 374
Corrueeini, R.J. 351(30) 354
Cowley, R.A. 6 102(12)
Craig, P.P. 371(79) 374
Craig, R.C. 351(26) 352(96) 354 355 370(24) 371(84) 372 374
Craighead, C.M. 282(38) 284 315(31) 316 327(35) 329
Crane, L.T. 351(55) 352(55) 354 370(48) 371(48) 373
Crawford, J.H. 175 183(86)
Crystal field potential 228
Crystal field potential, cubic, 4-fold coordination 236
Crystal field potential, cubic, 6-fold coordination 229ff.
Crystal field potential, cubic, 8-fold coordination 232ff.
Crystal field potential, cubic, operator equivalents 265ff.
Crystal field potential, cubic, spherical harmonic expression 269
Crystal field potential, magnetic ions 229ff.
Crystal field potential, matrix elements 247ff.
Crystal field potential, spherical harmonics expression of 237ff.
Crystal Radii, Ions 74
Crystal Radii, Ions, alkali halides 90
Cubicciotti, D. 43 53 102
Cuprous oxide, structure 11
Daane, A.H. 301(23) 304 314(12) 315(12) 316 319 326(16) 328 332(7 10) 333(7 10 11) 334 337(10) 338(35 36 38 40 41) 339 340 344(13) 345(27 28 34 37 38) 346 347 363(7) 364(7 8) 365
Dalla Croce, L. 174
Dalven, R. 380(12) 381
Darby, J.B., Jr. 321
Darwin, G.E. 281(2) 283 288(3) 290 292(3) 294 314(2) 316 326(5) 328 337(3) 339
Dash, J.G. 371(79) 374
Daunt, J.G. 351(53 65) 352(102) 354 355 360 370(45 58) 371(89) 373 374 377
Davis, S.G. 344(11) 346
Dawton, R.H.V.M. 384(15) 385
Dayal, B. 315(32) 316
de Klerk, J. 288(12) 290 292(11) 294 314(15) 316 319
de Launay, J. 368 369 370(97) 371(97) 374 379
de Nobel, J. 351(75) 352(75) 355 360 370(43) 371(43) 373
de Wette, F.W. 42 112 113 116
Debye temperature, comparison of 386
Debye temperature, elastic constants 379ff.
Debye temperature, electrical resistivity 383
Debye temperature, elements 368ff.
Debye temperature, elements, Table 370 380
Debye temperature, Lindemann equation, relation 378
Debye temperature, rare-earth metals 376
Debye temperature, specific heat data 369
Debye temperature, temperature variation 368
Debye temperature, thermal expansion, coefficient 383ff.
Debye temperature, X-ray intensity data 387
Delbecq, C. 130 143 144 164 175(38) 177 180 181 182 209 220 221 222
Dempsey, E. 68 103 119
Dennison, D.H. 332(7) 333(7) 334 337(10) 339 344(13) 346
Deshpande, V.T. 314(24) 316 413(12) 416
Desirant, M. 352(94) 355 371(82) 374
DeSorbo, W. 378
Dever, D.F. 345(31) 346
Dever, J.L. 326(1) 328 337(1) 339
Dexter, D.L. 154 201
Dichroic absorption 140
Dichroism, Potassium chloride 212 216ff.
Dick, B.G. 6 102(13)
Dienes, G.J. 133 180
Dillon, J.F. 272
Dipole-dipole interaction 30
Dipole-dipole interaction, alkali halides 32
Dipole-quadmpole interaction, ionic crystals 33
Dipoles, optical, alkali halides 137
Ditmars, W.E. 337(18) 339
Dolecek, R.L. 352(104) 355
Doty, P.M. 55
Douglas, T.B. 39 326(1) 328 337(1) 339
Douglass, R.L. 351(57) 354 370(49) 373
Douglass, R.W. 326(29) 327(29) 328
Downey, J.W. 321
Doyle, W.T. 164
Dreger, L.H. 337(26 29) 338(29) 339 344(23 24) 345(24) 346
Dreyfus, B. 352(87) 355 371(77) 374
Driekamer, H.G. 184 206 207
Drowart, J. 344(9) 346
Druyvesteyn, M.J. 281(27) 283 288(21) 290
du Chaterier, F.J. 351(75) 352(75) 355 360 370(43) 371(43) 373
Du Mond, J.W.M. 321
Duerig, W.H. 130
Dulong — Petit law 366
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



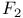 Center, energy levels, theory
Center, energy levels, theory 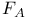 Center
Center 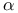 Center
Center