|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Rice F.O., Teller E. — The structure of matter |
|
|
 |
| Предметный указатель |
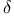 state 246 state 246
 state 246 state 246
 states 246 states 246
 functions 87 functions 87
 state 87 246 state 87 246
Accidental degeneracy 10 198
Acetylene, molecular vibrations of 226
Acoustical branch 218 229
Activation energies 75 76
Alkali halides, lattice energies 139
Alkali metals, chemical binding in 174 176
Alloys, structure of 178
Alpha particles 296 316
Alpha radioactivity 317
Aluminum phosphide, structure of 147
Ammonia, directed valence in 92
Anharmonicity 211
Anthracene, resonance in 120
Anti-Stokes line 223
Antibonding electrons 85
Aromatic compounds, electron mobility in 110 111
Atom, nuclear 1
Atomic models 235
Atomic Radii 142
Atomic spectra 231
Atomic-function method 71 75 85 95
Band spectra 254
Band system 254
Barn 298
Benzene, structure of 109 118 271
Beryllium hydride, shape of 95
Beryllium molecule, 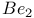 , stability of 86 , stability of 86
Beryllium, electric conductivity of 175
Beta radioactivity 305
Beta rays 305
Betatron 321
Binding energies in nuclei 316
Birge — Sponer method 260
Bismuth, diamagnetic susceptibility of 191
Bjerrum double band 208
Blue giants 337
Bohr magneton 184
Boltzmann constant 47
Boltzmann law 47
Bond, heteropolar 28
Bond, hydrogen 115 137
Bond, triple 100
Bonding electrons 85
Bonds, conjugated double 104
Born — Haber cycle 140
Boron molecule, 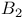 , binding energy of 89 , binding energy of 89
Breakdown, electric 172
Brillouin zones 164 168 171 173
Caesium chloride, structure of 141
Carbon cycle in stars 344
Carbon dioxide, molecular vibrations 196—198 206 208 210 214
Carbon molecule, 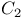 , binding energy of 89 , binding energy of 89
Carbon tetrachloride, vibrations of 215
Carbon-carbon double bond 100
Carbon-carbon triple bond 103
Carbonate ion, resonance in 114
Carboxyl group, resonance in 115
Catalysis, surface 180 181
Charge number 299
Chemical bond 28 71
Chlorophyll, light absorption and emission 285
Co-ordination schemes 247—253
Cobalt chloride ion, 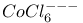 , shape of 143 , shape of 143
Cockroft machine 321
Color and resonance 272—278
Combination tones 213
Complementarity 4
Compound nucleus 324
Condon, Franck — Condon principle 255
Conduction electrons 170
Conductivity, electric 148 149 168
Conjugated double bonds 104—112
Conjugated systems, resonance in 116—122
Correspondence principle 5 205 274
Coulomb attraction 73
Curie point 189
Cyclotron 321
d state 12 232
Davisson — Germer experiment 3
Debye temperature 168
Decay constant 308
Deformation vibrations 210
Degeneracy 10 74 77
Degeneracy, accidental 10 198
Degeneracy, necessary 10
Degenerate states 9 233
Degenerate vibration 197
Depolarization factor 221 227
Deuterium, ortho-deuterium 303
Deuterium, para-deuterium 303
Deuteron 303
Diamagnetism 190
Diamond, structure of 146
dielectric constant 48
Diffraction, electron 41
Diffraction, neutron 42
Diffraction, X-ray 41
Dipole moments 46 52—57 203 206 208 211
Dipole radiation 201 234
Dipole structures in solids 134—137
Directed valences 92—94
Dissociation energies 89 90 257—262
Double bonds, conjugated 104—112
Double bonds, valence orbits 100—104
Doublet states 240
Dwarfs, red 337
Dwarfs, white 337
Dyes, organic 273
Electric breakdown 172
Electric conductivity 168
Electron affinities 29 139
Electron diffraction 41 42
Electron holes 122 123
Electron pair 91 92
Electron spin 34 35
Electron, antibonding 85
Electronic spectra 231—295
Electrons 1 2
Electrons in strong periodic fields 154—162
Electrons in weak periodic fields 151—154
Electrons of the K shell 20
Electrons of the L to Q shells 23—27
Electrons, bonding 85
Electrons, conduction 170
Elementary domains 188
Energy production, in stars 341—344
Energy, dissociation 257—261
Ethylene, vibrations of 226 230
Even state 233 247
Exchange integral 73
Excited state, first 8
Excited states, higher 12
Exciton 288
Expanding Universe 346—347
f functions 12
F state 233 273
f value 273
Fermi sea 167
Ferroelectricity 188
ferromagnetism 188 189
Fine structure 244
First excited state 8
Fission 316 319
Fluorescence 223
Fluorine molecule, 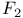 , binding energy 88 122 123 , binding energy 88 122 123
| Fourier series 212
Franck — Condon principle 255 270
Free rotation 230
Fundamental state 7
Fundamental tone 212
Gamow formula 318
Geiger — Nuttal relation 319
Germer, Davisson — Germer experiment 3
Giants, blue 337
Giants, red 337
Graphite, structure of 121 128 177
Group of waves 161
Group theory 232 239 267
Group velocity 161
H states 13—16
Hall effect 169 170
harmonic oscillator 194
Harmonic vibrations 205
Hartree model 235 238 242 246 247
Heitler — London model 71 74 78
Helium ion, 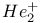 , structure and binding energy 85 86 , structure and binding energy 85 86
Helium, solid 174
Heteropolar bond 28 29
Hume — Rothery rule 179
Hund — Mulliken method 78 91 247
Hund's case a 265
Hund's case b 265
hybridization 96 97 145
Hybridized functions 94—99
Hydrogen atom 7
Hydrogen bond 67 137
Hydrogen bridge 67 116 137
Hydrogen bromide, dipole moment of 55
Hydrogen chloride, dipole moment of 54 55 201
Hydrogen iodide, dipole moment of 55
Hydrogen ion, hydrated, 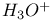 113 113
Hydrogen molecule ion, 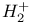 , structure of 80 , structure of 80
Hydrogen molecule, structure of 74 75 84 85 92 115 248
Hydrogen, metallic 174
Hydrogen, solid, conductivity of 173 174
Hydroxyl bond 134 137
Hyperfine structure 233
Ice, structure of 134
Indicators, acid-base, theory of 277
Infrared spectra 193 200 205 216
Instability of stars 345
insulators 166 167 292
Internal conversion 333
Internal photoeffect 292
Interstitial structures 179—180
Iodine molecule, size 134
Iodine spectrum 259
Ion interactions 68—70
Ionic complexes 142—145
Ionic crystals 137—142
Ionization energies 29
Isobars 300
Isomers 309 333
Isotope effect 39 40 228
Isotopes 300
K shell, electrons of 20
Kekule formulae 117—121
Kekule structures, electronic spectra of 271
Keto-enol isomerism 116
L shell electrons 23
L-S coupling 240 253
Lattice energies of alkali halides 139
Linear accelerator 321
Liquids, infrared spectrum of 216
Lithium molecule, 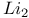 86 87 86 87
Lithium, metallic, structure and conductivity of 174
London forces 66
Low temperatures, magnetism and production of 187
magnetic moment 183 184 300
Magnetic moment of neutrons and protons 300
Magnetic properties of matter 182—192
Main sequence stars 337
Mass number 299
Mathematics, use of 1
Maxwellian velocity distribution 208
Meson 336
Metals 147 166
Methane, shape of 99
Methyl radical 97
Methylene group 96
Molecular rotation 199—200
Molecular vibrations 193
Molecular-orbital method 76—78 85 92 104—111
Moment of inertia 199 203 208 209
Multiplicity of states 240 242 253 266
Naphthalene, structure of 110 111
Necessary degeneracy 10 197
Neon molecule, 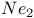 , binding energy in 88 , binding energy in 88
Neutrino 307
Neutron 298 299
Neutron diffraction 42
Neutrons, fast, reaction of 329
Neutrons, production of 325—328
Neutrons, slow reactions of 330—333
Nickel atom, energy levels of 24
Nickel cyanide ion, 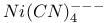 , shape of 144 145 , shape of 144 145
Nitric oxide, paramagnetism of 185
Nitrite ion, shape of 144
Nitrogen molecule ion, 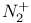 , binding energy in 89 , binding energy in 89
Nitrogen molecule, vibration of 201 220
Nitrophenol, para-, resonance in 118
Nitrosyl chloride, shape of 144
Normal vibrations 194—198 206 210 213 218 229 270
Normalization factor 8
novae 345
Nuclear atom 1
Nuclear fission 319
Nuclear forces 335
Nuclear isomers 332
Nuclear resonance 324 325
Nuclear stability 312—315
Nuclear vibrations 39
Nuclei, size of 298
Nucleus, compound 325
Nuttal, Geiger — Nuttal relation 319
Odd states 232 246
One-electron spectra 237
Optical branches 218
Organic dyes, color and resonance 274—278
Origin of elements 347
Ortho-deuterium 303
Ortho-helium 242
Ortho-hydrogen 300
Orthogonality of wave functions 9—10
Oscillator, harmonic 193
overtones 212
Oxygen molecule ion, 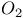 , binding energy of 89 , binding energy of 89
Oxygen molecule, binding in 88 102
Oxygen, paramagnetism of 185
P branch 207 208
p state 11 233
P states 233
Palladium, hydrogen in 179
Para-helium 242
Para-hydrogen 300
Para-nitrogen 304
Parallel bands 209 253 263 267
Parallel transitions 253
Paramagnetism 185 190—194
Parity 232
Pauli principle 21 35 237 239 240 242 300 304
Penetration factor 322
Periodic system 22 315 316
Perpendicular bands 209 253 263 267
Perylene, resonance in 111
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



 state
state  state
state  states
states  functions
functions 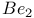 , stability of
, stability of 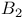 , binding energy of
, binding energy of 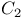 , binding energy of
, binding energy of 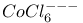 , shape of
, shape of 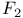 , binding energy
, binding energy 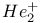 , structure and binding energy
, structure and binding energy 
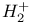 , structure of
, structure of 
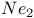 , binding energy in
, binding energy in 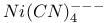 , shape of
, shape of 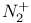 , binding energy in
, binding energy in 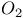 , binding energy of
, binding energy of