|
|
 |
| Авторизация |
|
|
 |
| Поиск по указателям |
|
 |
|
 |
|
|
 |
 |
|
 |
|
| Verwey E.J.W., Overbeek J.Th.G. — Theory of the stability of lyophobic colloids |
|
|
 |
| Предметный указатель |
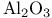 -sol 9 -sol 9
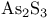 -sol 8 -sol 8
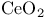 -sol 11 -sol 11
 -sol 9 -sol 9
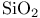 -sol 111 -sol 111
Adherence of quartz part-icles to a plane wall 185
Adsorption potential .adsorption theory, Freundlich’s 9
Aggregation, probability of 164
AgI-sol 4 8
Amicronic sols .anisodimensional particles 126
Aschenbrenner, M. 11
Attraction between spherical particles 160
Attractive forces 98
Attractive potential for two plates 101
Au-sol 8
Bear.R.S. 13
Bergmann, P. 13 21 90 106 187
Bernal.J.D. 12 13 125
Boltzmann’s theorem 23
Born-repulsion 163 181
Born.M. 50
Brownian movement 166
Bungenberg de Jong, H.G. 3
Casimir, H.B.G. 23 60 105 185
Chapman, D.L. 22
Charge of the double layer 30
Charge, surface 5 74
Charging methods, equivalence of two 63
chemical potential 47
Coagulation, time of 167
Coagulation, velocity 166
Collisions, number of 166
Colloids, irreversible 2
Colloids, lyophilic 1
Colloids, lyophobic 1
Colloids, reversible 2
Corkill.A.J. 21 67 188
De Boer.J.H. 14 15 17 20 100 195
De Bruyn.H. 46
De Smet, M. 49
Debye.P. 22 58 98
Derjaguin, B. 21 32 52 90 136 137 158 167 187 189
Diffuse charge layer according to the theory of Gouy — Chapman 28
Dilatancy 14 15
Discharging process with constant surface potential 57
Double layer 6
Double layer around a spherical particle 37
Double layer at the interface of two liquids 34 49
Double layer interaction 51 60 66
Double layer with constant charge 62
Double layer, charge and potential in the electrochemical 22
Double layer, diffuse 5
Double layer, flat 25
Double layer, free energy of the 54 58
Double layer, single 1
Double layer, Stern’s theory for a flat 41
Double layers, interaction of two double diffuse 75
Dube, G.P. 21 92 135 143 146 152 188 189
Einstein, A. 166
Electro-endosmosis 48
Electro-kinetic phenomena 49
Electrocapillary curve 55
Electrodeposition of fine suspensions 17 178
Electrolytes, indifferent 7
Electrophoresis 48
Electroviscous effect 3
Emde, F. 69
Emulsifiers, two kinds of 134
Emulsions. 18 34 133
Energy and free energy of the double layer 190
Energy of interaction (two spheres), total potential 160
Energy, configurational 190
Ettisch, G. 9
Fankuchen, I. 12 13 125
Flat plates, attraction between two 100
Flat plates, interaction of two parallel 66
Flocculated systems 108
Flocculating concentration 117
Flocculation values 8 9 172
Flocculation velocity 166
Free energy of a double layer system 51
Free energy of the double layer 54 58
Free energy, chemical part of the 53
Free energy, electric part of the 53
Freundlich, H. 8 9 13 14 125 126 130
Fuchs, N. 164
Gelation 10
Gibbs — Helmholtz equation 192 193
Goodeve, C.F. 12
Gouy — Stern layers, charges in the combined 129
Gouy, G. 22
Gouy-layer 42
Green’s Theorem 60
Gundermann, J. 13
Gyemant, A. 186
Hamaker, H.C 17 20 100 103 121 125 160 178 180 181 195 198
Hauser, E.A 12
Heller.W. 12 13 125
Hess, K 13
Hiickel, E. 22 56
hydration 2
Interaction of colloidal particles 20
Interaction of double layers 6
Interaction of two spherical double layers 149
Interaction, energy of .for large particles, when 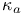 is small 143 is small 143
Interaction, energy of for large particles with a thin double layer 137
Interaction, small 70
Interaction, strong. 71
Interface liquid/liquid. 75
Ishizaka, N. 9
Jacobson, K. 13
Jahnke, E. 69
Joachimson, K. 9
Jonker, G.H. 178
Juliusburger, F. 126
Kallmann.H. 20
Keesom.W.H. 98
Kiessig.H. 13
| Kinetics of coagulation 164
Kirkwood, J.G. 23 99
Klompe, M.A.M. 8
Kruyt, H.R. 1 3 4 8 9 11 12 46 167 176 178
Kussakow, M. 21
Langmuir, I. 19 21 42 90 187 195
Langmuir’s method of discussing stability. 195
Laplace operator 23
Levine, S. 21 92 135 143 146 152 188 189
Lewis, W.C.M. 186
Lippmann equation 55
London — Van Der Waals forces, relativity correction to the theory of 104
London, F. 19 98
London-Van Der Waals attraction forces 19 98
London-Van Der Waals constant A 121
Long-range attractive forces 19
Low-Beer, P. 13 21 90 106 187
Lyotropic influence. 181
Macromolecule 2
March, A. 186
Miiller, H. 38 39 131
Minimum in the potential curves for great distances 124 182
Moorkens, M . 49
Morawitz, H. 8
Moulding 16
Neugebauer, Th. 99
Niessen, K.F. 25 34
Osmotic pressure 196
Overbeek, J.Th.G. 21 49 126
Palmer, K.J. 13
Particle radius, influence of on stability 176
Particles, anisodimensional 126
Philippoff, W. 13
Plasticity 15 16
Poisson’s equation. 23
Polder, D. 105 185
Postma, J. 11
Potential curves 20
Potential determining ions 46
Potential energy curve, influence of the concentration of electrolyte on the 115
Potential energy curves for two flat plates 110
Potential energy for two flat double layers 76
Potential energy, total 106
Quimfe, G. 12
Recondary minima in the energy curves 184
Reitstotter, J. 167
Repeptization phenomena 181
Repulsion for intermediate values of  157 157
Repulsive potential 81 83
Repulsive potential between two spherical particles 140
Repulsive potential, influence of the concentration of electrolytes. 86
Repulsive potential, influence of the valency 88
Repulsive potential, via the force 90
Retardation effects on the London — van der Waals forces 105 183
Rheopexy 125
Rice, O.K. 186
Roder, H.L. 14
Rosenhead, L. 21 67 188
Rutgers.A.J. 25 49 50
Schiller-layers 90 188
Schmitt, F.O. 13
Schulze and Hardy, rule of 7 119 173 188
Sediment, plastic 15
sediment, volume of the 15
Slater, J.C. 99
Slipping-plane 48
Small particles, low stability of 171
Spherical colloidal particles, interaction of 135
Spherical particles, charge and potential of two interacting 144
Spontaneous repeptization 181
Stability 6
Stability conditions, graphic survey of the 115
Stability curves 121
Stability of colloids 164 169
Stability of emulsions 133
Stability of small particles 124
Stability with respect to “coarsening” 186
Stability, colloid 107
Stability, influence of thermal motion of particles 123
Stable systems 108
Stauff, J. 13
Stern correction for spherical particles 180
Stern correction on the interaction of two flat plates 126
Stern equation 43
Stern theory, flocculation values according to the 132
Stern, O. 41 127
Stern-layer 42
Streaming-potentials 48
Structure of the soil 17
Surface charge (function of plate distance) 74
Surface potential 46
Suspensions 13
Suspensions, mechanical and rheological behaviour 10
Suspensions, stable and flocculated 14
Swelling and shrinking, general discussion on 189
Tactoids 13 125
Thickness of the diffuse layer 27
Thixotropic gelation 125
Thixotropy 10
Tobacco mosaic virus 13 125
Total potential energy, curves of 161
Troelstra, S.A. 176
Two layers, separation into 196
Valency of ions, influence on flocculating concentrations 119
van Arkel, A.E. 167 178
Van Der Made, J.E.M. 11
Van Der Minne, J.C. 134
van der Waals — London attraction forces see “London”
Van Laar, J.J. 92
Verlende, Ed. 25
Verwey, E.J.W. 4 9 14 15 15 17 21 25 34 46 50 72 76 126 131 133 178
Von Buzagh, A. 185
Von Elissafoff, G 8
Von Smoluchowski, M. 164
Westgren, A.Wijga, P.W.O. 49
Willstatter, M. 20
Zeta potential 46
Zocher, H. 13 21 90 106 187
|
|
 |
| Реклама |
 |
|
|
 |
|
О проекте
|
|
О проекте



 -sol
-sol  -sol
-sol 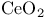 -sol
-sol  -sol
-sol 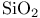 -sol
-sol 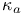 is small
is small